Measuring corrosion.

Anecdotal information and personal opinions are valuable starting points of inquiry.
This picture shows me making observations and measurements in the 1970's.
The first requirement when studying any subject is to be able to compare observations made during a period of time. This is why we must note the time at which observations are made. We must then be able to describe the observations and record them accurately.
This can now be done using video cameras, photographs and recording electrical data gathered by sensors
and electrical devices that display the results visually.
Engineering requires that actions must have known results that can be repeatedly demonstrated. We can therefore make designs that will benefit humankind and that will last as long as required.

Corrosion is electrochemical and we can make accurate measurements of electricity in everyday life. We can now make accurate measurements of chemistry in laboratories using sophisticated instrumentation that is not portable.

We are gathering electrical data that we are trying to analyse with respect to corrosion in an effort to control corrosion on a large scale. Corrosion related failures are occurring globally with catastrophic results. We need to improve our understanding and control of corrosion. Misinformation about the measurement of corrosion has been a major part of the marketing of corrosion control services for over 40 years.
The first requirement when studying any subject is to be able to compare observations made during a period of time. This is why we must note the time at which observations are made. We must then be able to describe the observations and record them accurately.
We already measure corrosion cells very accurately in order to make our computers work as batteries are essential components of modt electronic devices that run our global community.
Engineering requires that actions must have known results that can be repeatedly demonstrated. We can therefore make designs that will benefit humankind and that will last as long as required.
Corrosion is electrochemical and we can make accurate measurements of electricity in everyday life. We can now make accurate measurements of chemistry in laboratories using sophisticated instrumentation that is not portable.
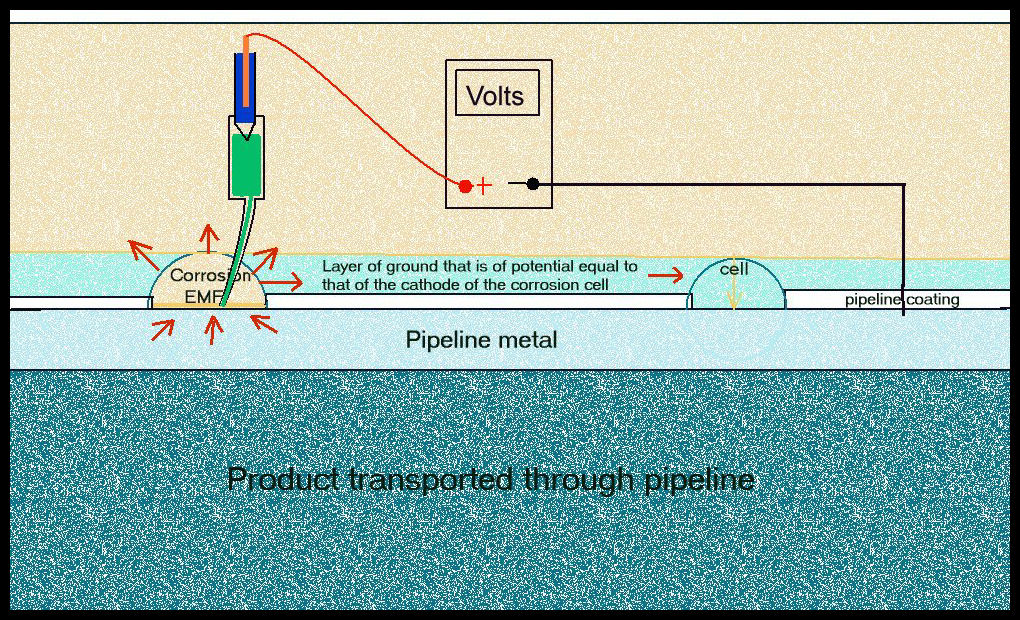
How to measure corrosion in a corrosion cell
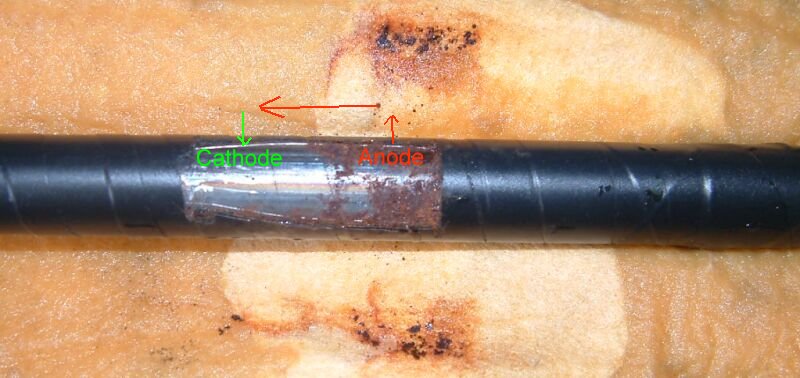
A corrosion cell generates electrical charges by the reaction between a chemical and a metal. If these charges can circulate, their energy can be used in the form of work. If they do not circulate, metal does not dissolve and the system is ‘in equilibrium’ without corrosion taking place. In this condition the metal is not damaged.
The anode of a corrosion cell discharges electricity as the metal of the anode dissolves. The metal at the cathode of the system does not dissolve as the charges re-enter the metal to complete their circuit.
The difference in electrical charge between the anode and the cathode of a system can be measured as a 'potential difference', known as a volt, and displayed on a meter in the form of a visual display.
We therefore need to know the way in which this display is achieved in order to understand what the readings mean.

A potential is an electrical quality that every item has in relation to the electrical quality of all other items in the universe. This quality is the potential to do work and is a form of energy. In a corrosion cell we can measure this quality in relation to the system of which it is part. We do this all the time in electronics, otherwise our devices would not work and we would not have mobile phones or televisions.

The corrosion cells with which we are all familiar are known as batteries. We manufacture batteries for specific purposes and they are designed to have specific energy outputs that are measured in volts using voltmeters. Voltmeters measure the potential difference between the anode and the cathode of each battery or the combined effect of many anodes and cathodes arranged in 'series'.











