Members of CPN cannot afford to offer our services for free as we need to live well to function as engineers and scientists.
Facebook is free software and therefore it gives the opportunity for those of us who have little or no money to structure ourselves to offer a service to those who have money and control the world.
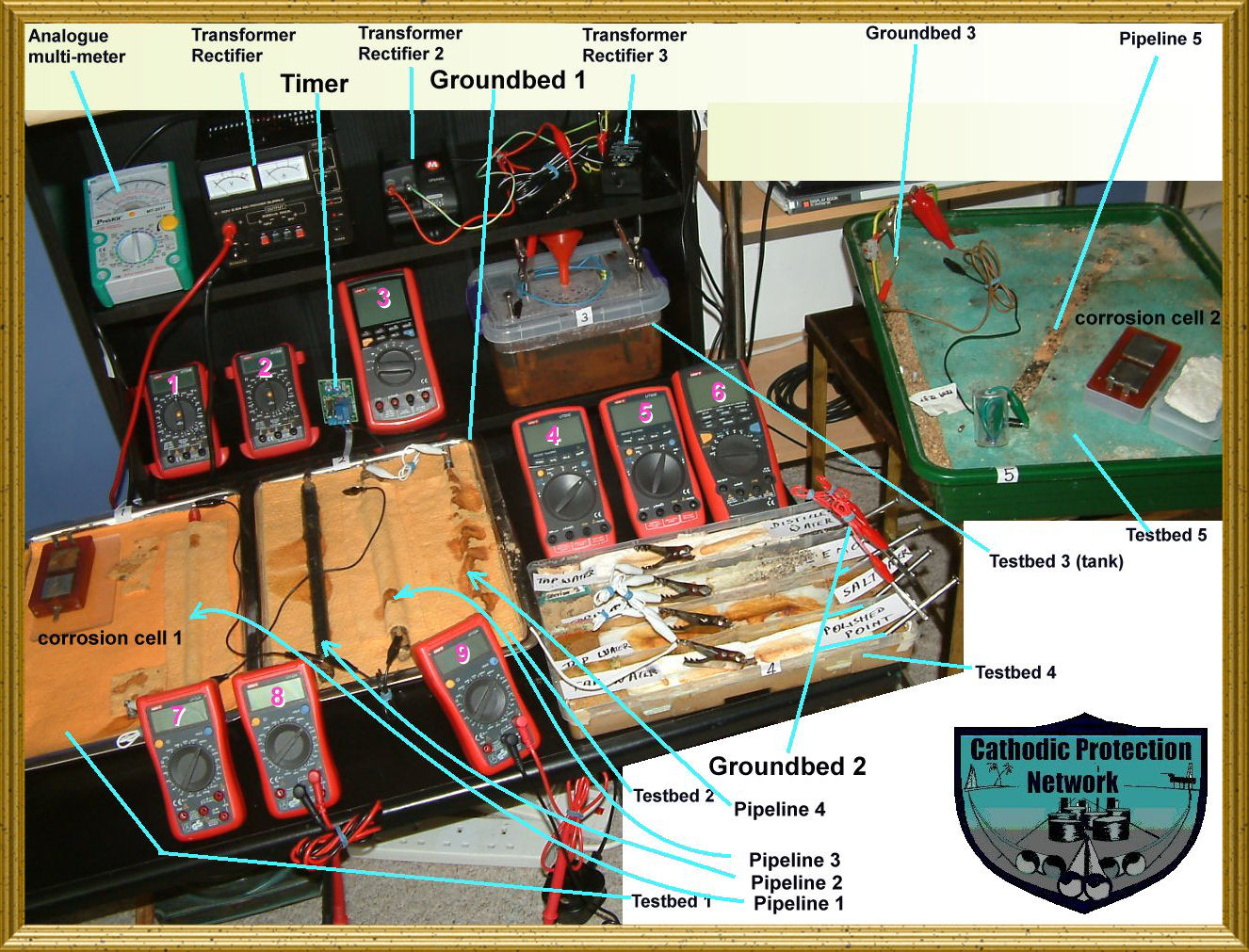
CPN is a structure using the internet to control corrosion in the pipeline, oil, gas and water distribution industries.
Corrosion control has failed because individuals are competing instead of cooperating when in reality we are competing with nature irself that is trying to reduce all materialsback to their original state.
A blockchain is a computer program that allows the value of work to be added to any block in the chain adding to the value of every block in the chain. The computer uses this protocol to to decide how much the owner of each block can draw value (in currency) from the blockchain
Followers of CPN can join the blockchain structure that allows us to cooperate without any competition because it removes the problem of trust.
Those wishing to be involved in this global project should email me at
roger.alexander3@ntlworld.com
and I will then enter them into my admin system in the folder of the country in which they are resident.
I will then send them instructions about the structure of Cathodic Protection Network and how it functions financially, technically and scientifically in the only way that can really control corrosion to networks of pipelines and facilities globally.
Those who have simply clicked to join the coordinators group have not put any work into the blockchain and will therefore get no money out.
|