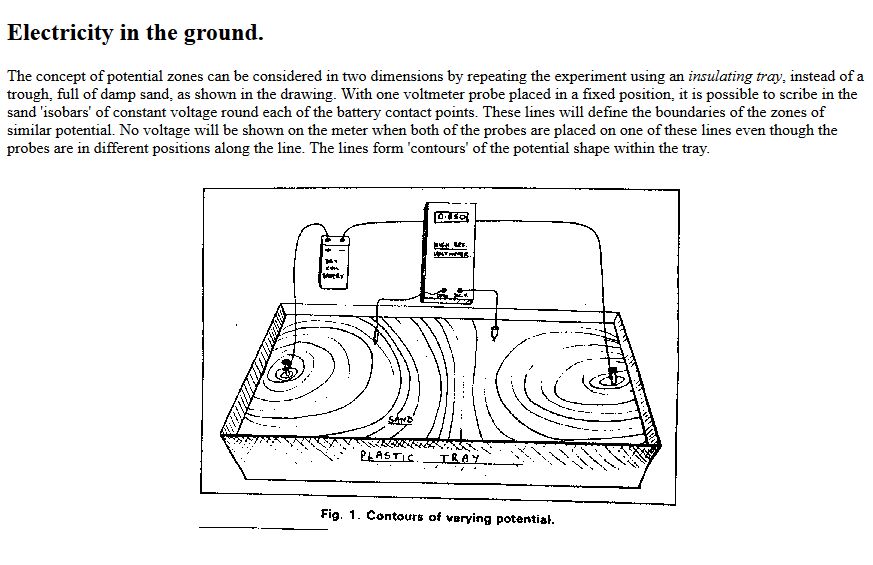
CATHODIC PROTECTION NETWORK INTERNATIONAL
74 DALCROSS
BRACKNELL
BERKSHIRE
RG12 0UL
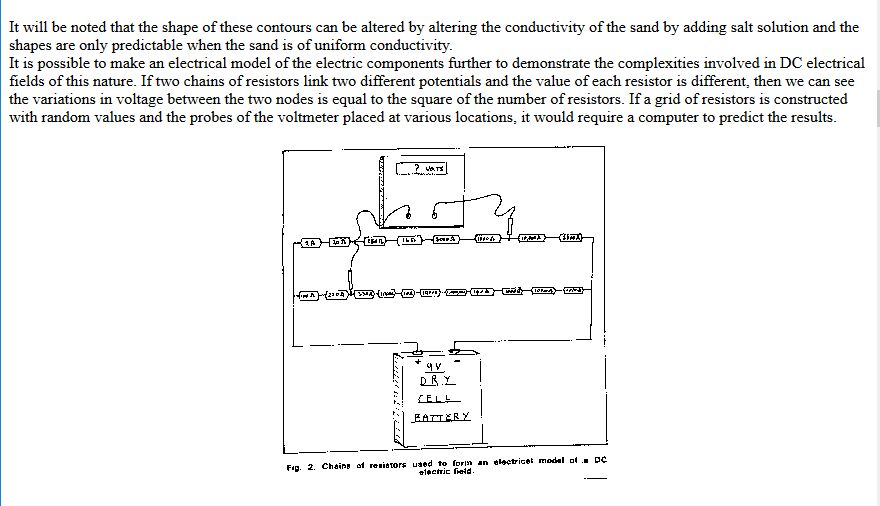
Cathodic Protection
The scientific theory of cathodic protection is that we must lower the electrical energy of the pipeline metal to a level at which it cannot corrode.
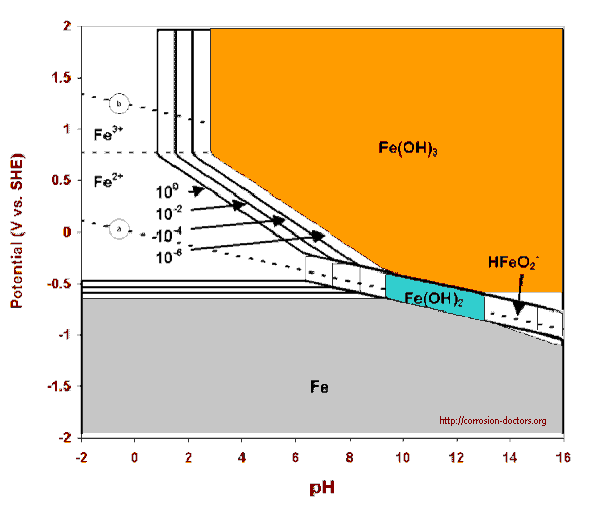 Scientists believed that the works of Pourbaix substantiated the use of a standard voltage -0.850v when measured between the pipeline metal and a copper/copper sulphate electrode as the criterion for achievement of cathodic protection.
Scientists believed that the works of Pourbaix substantiated the use of a standard voltage -0.850v when measured between the pipeline metal and a copper/copper sulphate electrode as the criterion for achievement of cathodic protection.
Faraday showed that energy is discharged in proportion to the weight of the metal dissolved in the corrosion process.
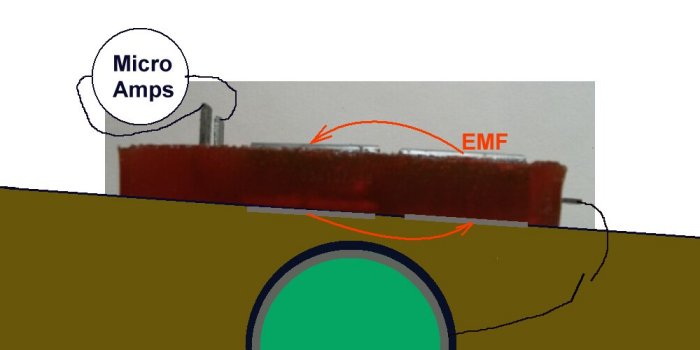
If we can control the amount of energy being dicharged we can control the corrosion.
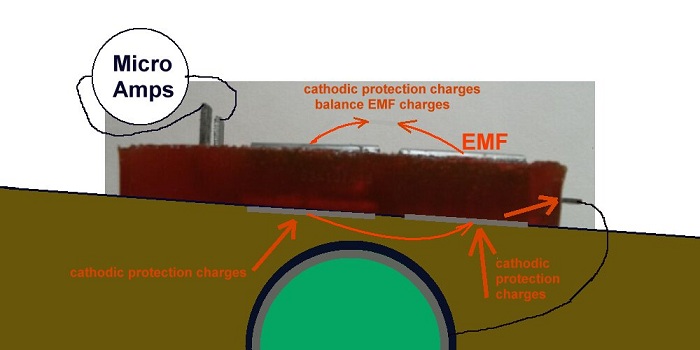
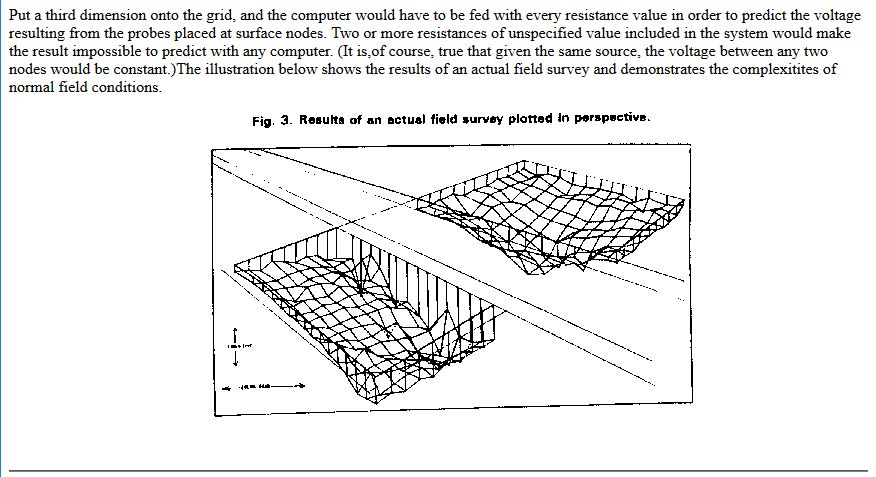
If a steel pipeline is coated with insulation, it separates the metal from the ground (electrolyte) and this stops the chemical interfacing with the metal.
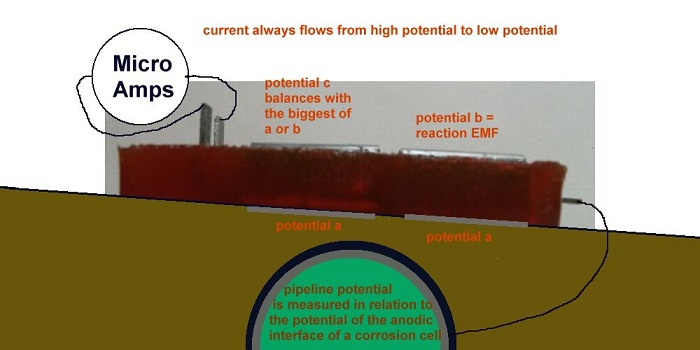
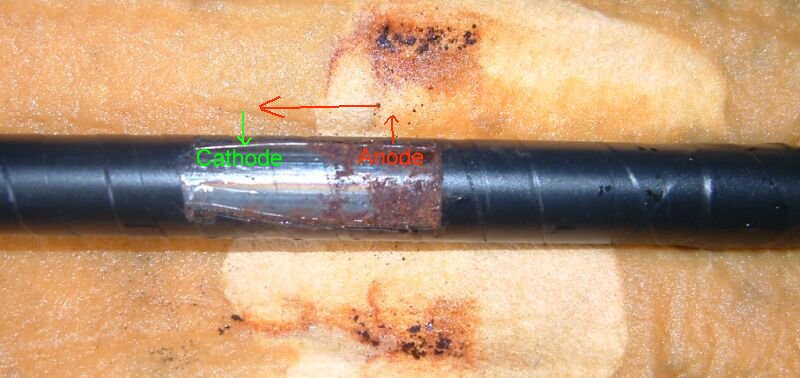
No coating is perfect so we must address the coating faults and these can either be Anodes (where energy leaves the metal) or Cathodes (where energy enters the metal) or both anode and cathode.
At this stage it is essential to understand the nature of electrical energy and this is governed by the laws of physics including Gibbs Free Energy that states that there cannot be no energy because "free energy" is everywhere in the universe and will fill any void.

I explain this in this video
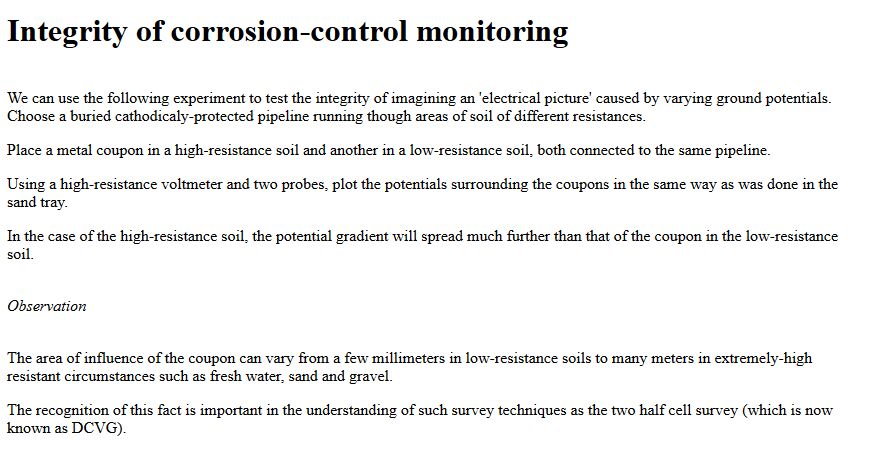
This means that there cannot be negative energy, all levels of energy are relative. This is why we measure the DIFFERENCE between two 'potentials' and call the measurement a volt.

This means that we have to lower the energy in the pipeline relative to the energy in the electrolyte at the anode of each coating fault. It is very important that all cathodic protection technicians, engineers and scientists remember this scientific fact.

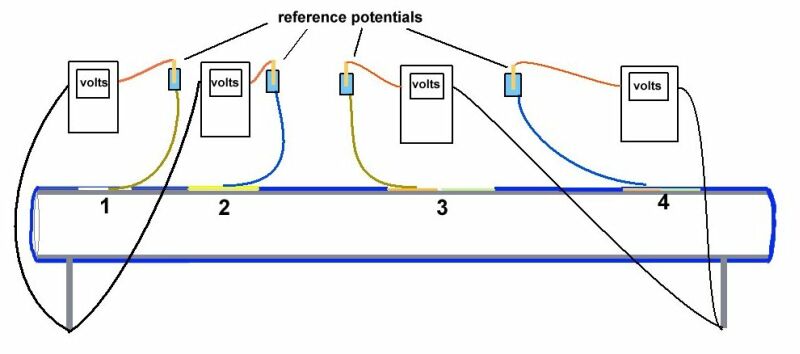
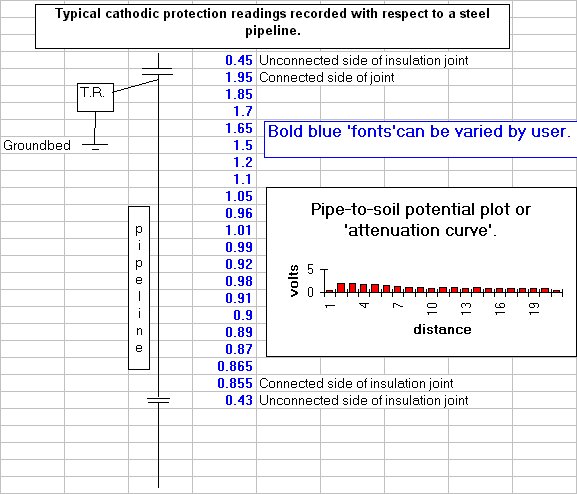
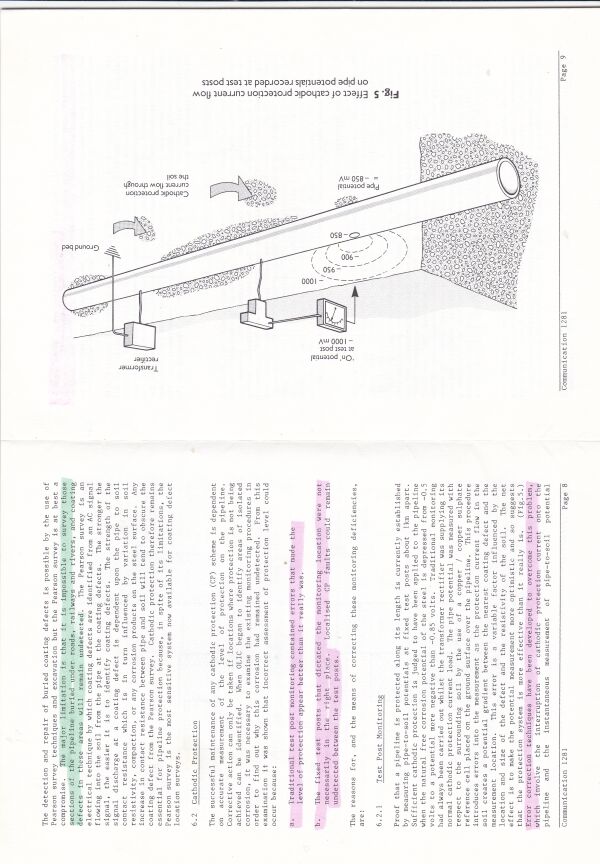
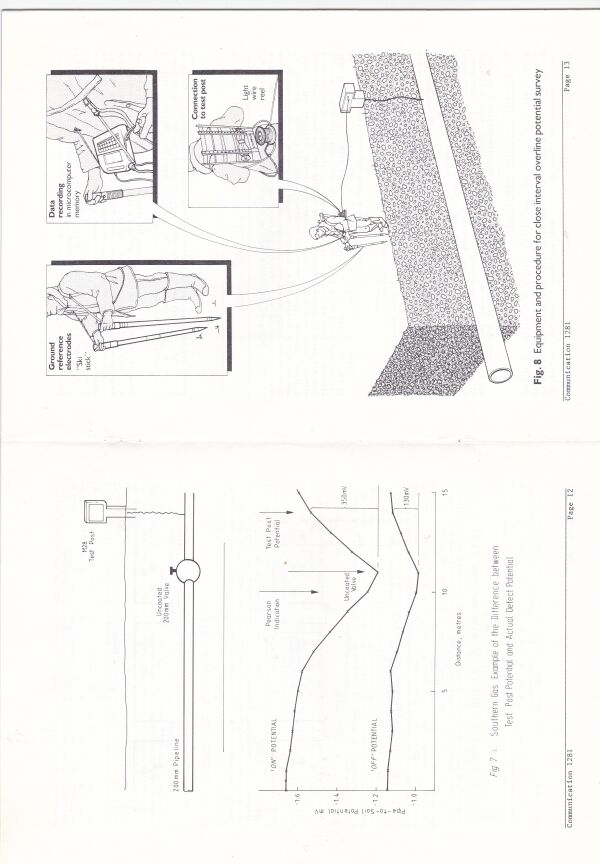
Cathodic protection test leads are connected to the pipeline metal at intervals varying from half kilometer to one mile, depending on the country and operators specification. Additional test points are provided at locations where the pipeline passes beneath roads, railways, canals and rivers.
The test leads are very well insulated because they are copper and would tend to form a 'bi-metalic coupling' reaction causing accelerated corrosion to the steel pipe.
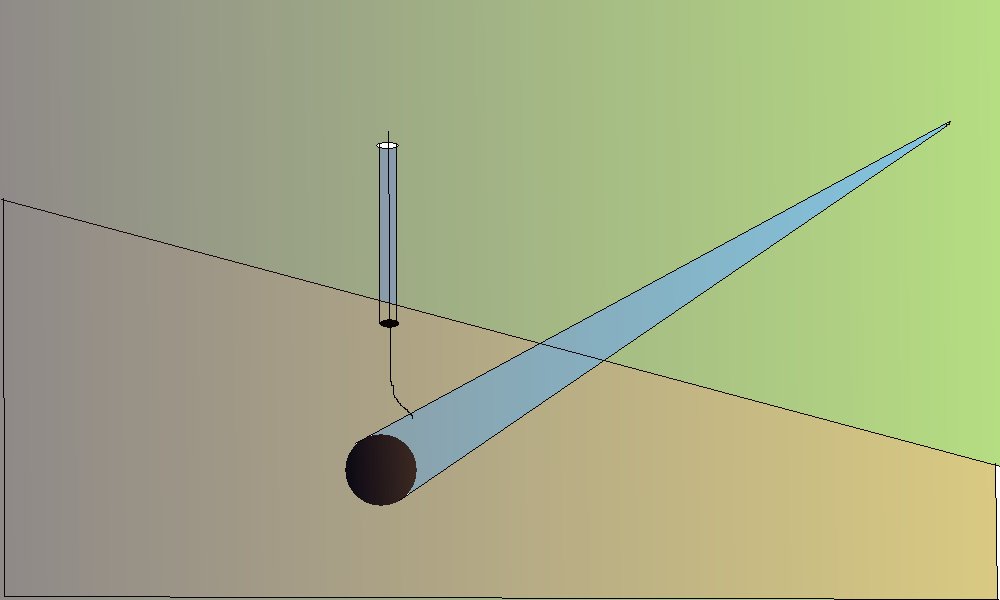
The test leads are bought to the surface through a pipe or concrete post set in the ground. The ends are normally attached to brass nuts and bolts which protrude giving access for electrical connection to instruments.

Vandals often damage these test posts so it is sometimes necessary to simply use a steel post and set the lead in concrete or epoxy compound to the top of the post.

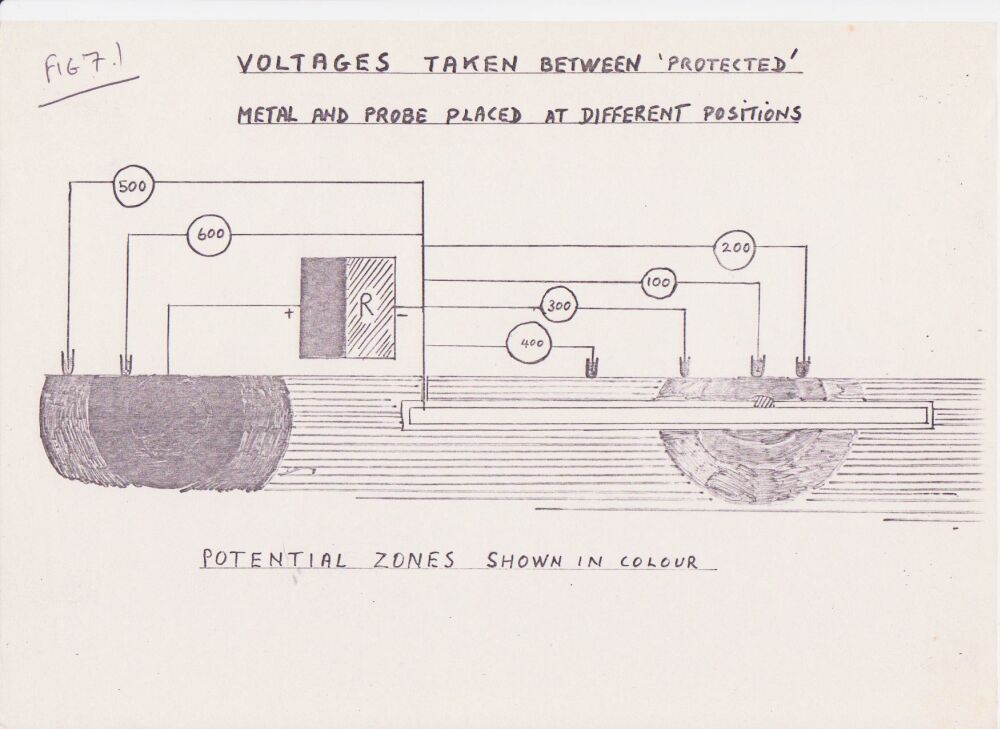
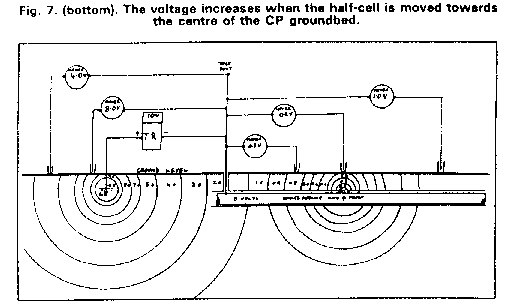
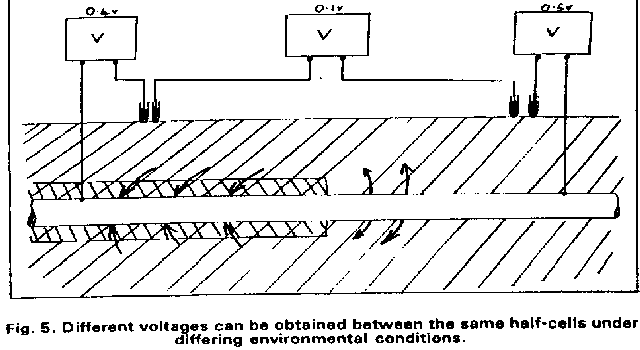
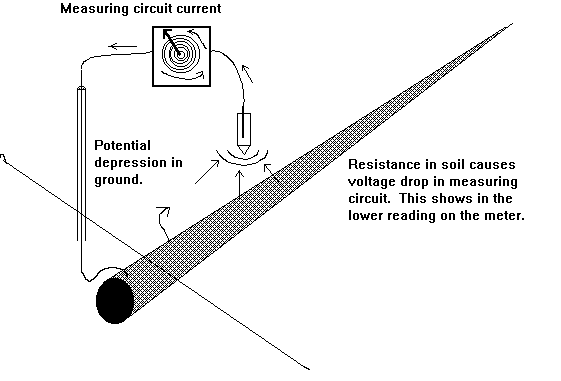
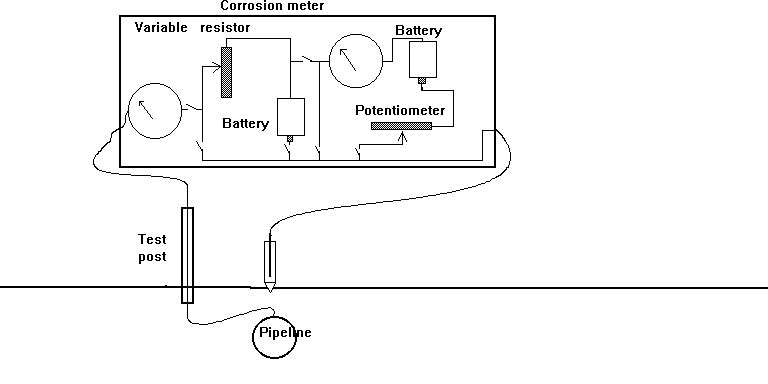
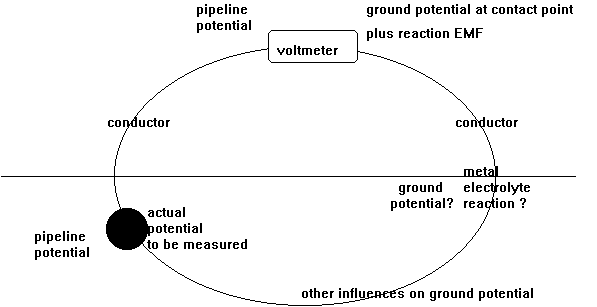
Cathodic protection Inspectors were required to connect the test lead to the negative terminal of a voltmeter, connect the positive terminal to an electrode described as a 'half-cell' which was placed on the ground directly above the pipeline.

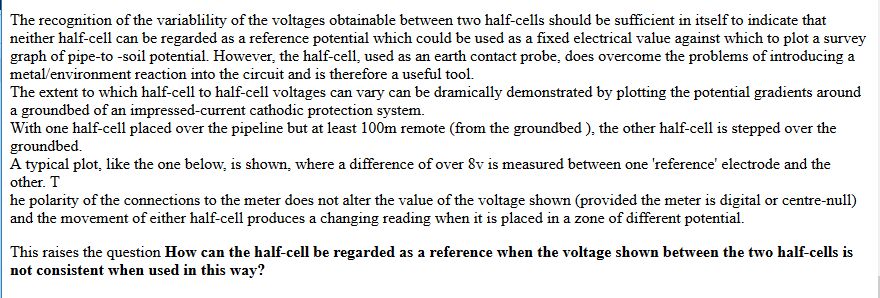
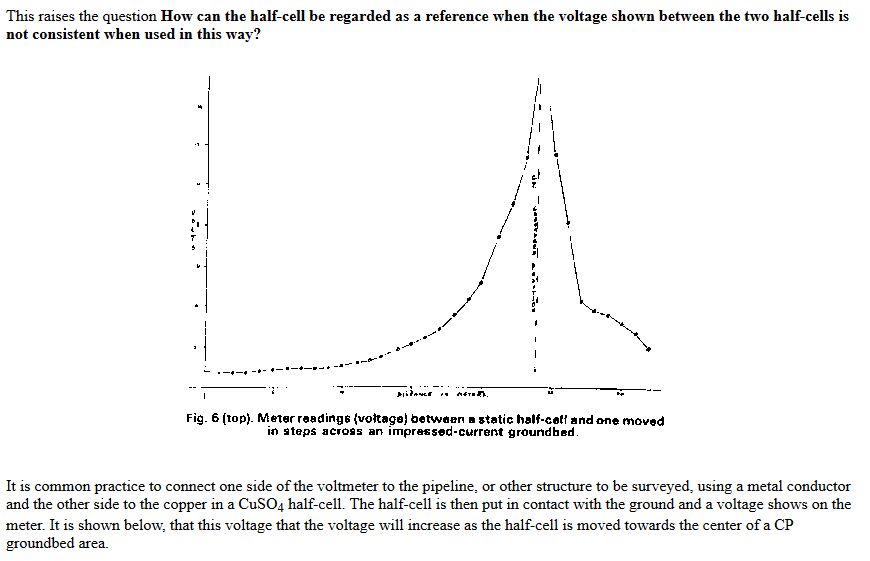
It was thought that the half-cell was a reference potential against which it was possible to measure the voltage which would give an indication of the corrosion status of the pipeline metal.
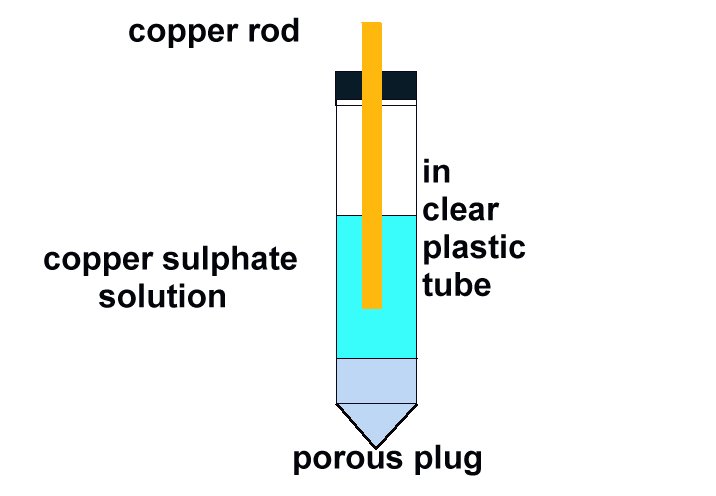
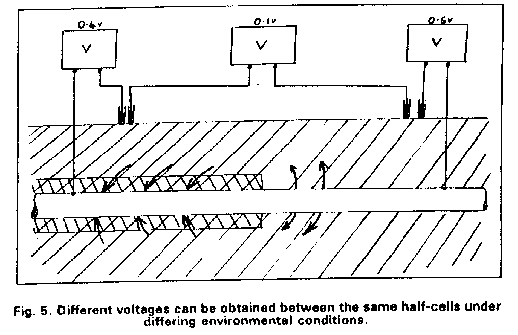
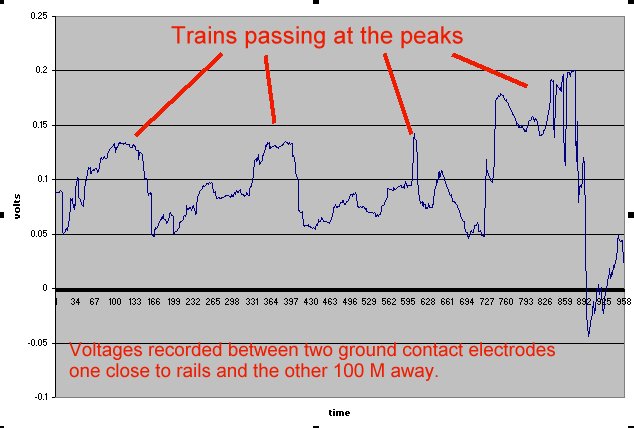
Field workers found that moving the half-cell would produce a significantly different result and when they reported this the data was altered to suit the expectations of the clients consultant engineers.
The reason why this electrode was called a half-cell was because of a misunderstanding of a laboratory procedure that is used to measure corrosion reactions.
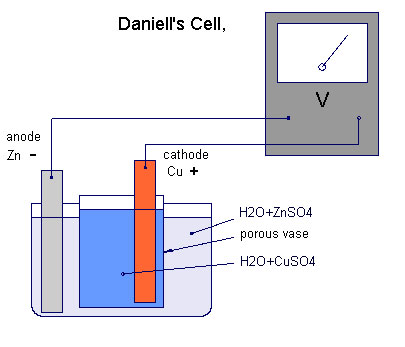
It was thought possible to take half of this 'Daniell Cell' as a reference potential as it's reaction electromotive force is known as a 'half cell reaction'.
This is not possible because the whole set up in real science is known as a potentiometer because it measures potentials.
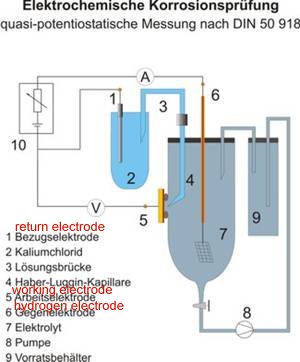
This is in a 'closed circuit' isolated from external electrical influences and temperature controlled in a laboratory.
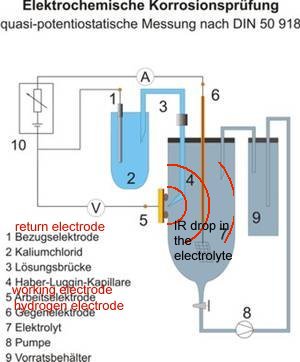
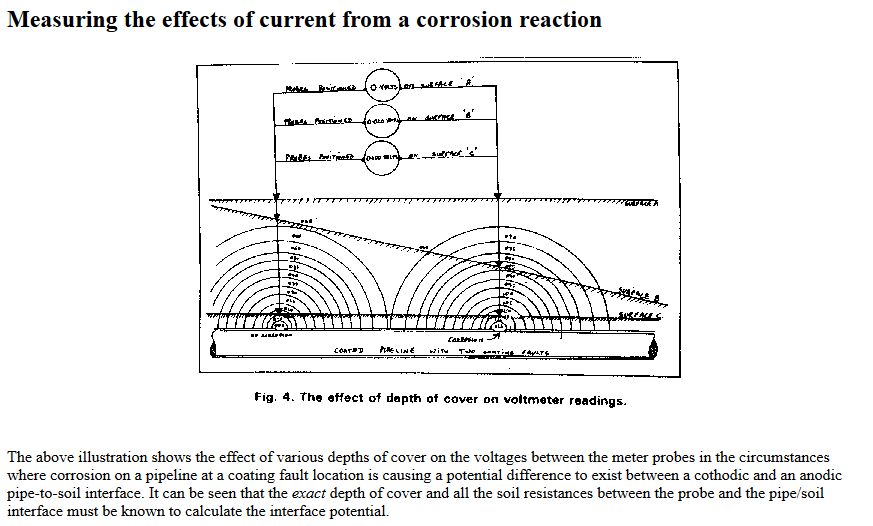
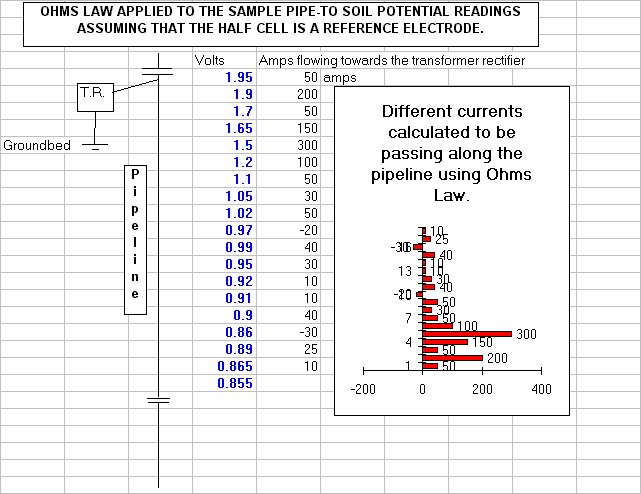
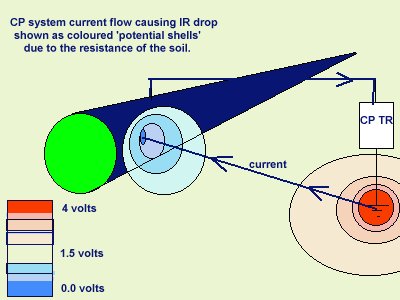
You will see that I have added to the drawing the 'IR Drop' as this expression is specifically used to describe a value that cannot possibly be measured. This term has been misused in the CP community to mean the potential gradient caused by the passage of electrical current through a resistance. The potential gradient in the ground is measured in DCVG and CIPS surveys all over the world.

From the 1950's to the 1970's, the source of scientific excellence and engineering guidance was a publication known as 'Peabodies' published by the National Association Of Corrosion Engineers.
Although cathodic protection had been a very cost effective success there continued to be disastrous corrosion related pipeline failures world wide and in the mid 1970's the method of making the field measurements was closely examined.
History of development of
the Cathodic Protection Network
I first entered the pipeline construction industry as an industrial safety officer in 1965. Until 1968 I studied the construction of pipelines and all safety aspects including the electrochemical aspects of cathodic protection with respect to earthing and spark control in intinsicaly safe areas.
Pipelines are mainly made of steel and coated with material that protects them from chemical reaction with their backfill. This coating must be electrically resistant and is inspected immediately before the pipeline is buried or submerged.

Steel pipes leave the factory in lengths of about 40 feet which are welded together into continuous lengths before lowering into the ditch. Each length has been factory coated but the gaps left for welding are coated in the field.

I 1973 I was certified as a coat and wrap inspector at British Gas ERS and then as a Cathodic Protection Technician on North Sea Gas pipelines in the UK.
Coating inspection is known as 'holiday detection' from the old expression of paint inspectors that the painter had taken a holiday when leaving a bare patch. The primary inspection is visual plus a continuous spring ring is wrapped round the pipe and rolled along using an insulated handle which connects the spring to a very high voltage coil.
The voltage is set so that an arc occurs at a coating defect and rings a bell in the detector box. The inspector marks the fault which is repaired and re-checked.

The detection and repair of coating faults delays the work of pipe laying which involves using heavy plant and equipment in difficult circumstances, all of which make it very easy to damage the coating further.

It is not surprising that coating faults are not uncommon.
The back-fill operation is inspected but often includes metalic and hard objects that can effect the cathodic protection measurements and physical condition of the pipeline when covered.
I found a scrapped car buried at Hedgerly Manifold on the North Thames Gas high pressure gas transmission system. It had been there for years and nobody else had detected it because they did not understand the science that supports cathodic protectin engineering.
In 1974 I was appointed to the position of Corrosion Engineer for the Eastern Division of Shell-BP Development Corporation of Nigeria.

It was very clear that corrosion control was not effective as leaks were increasing alarmingly.
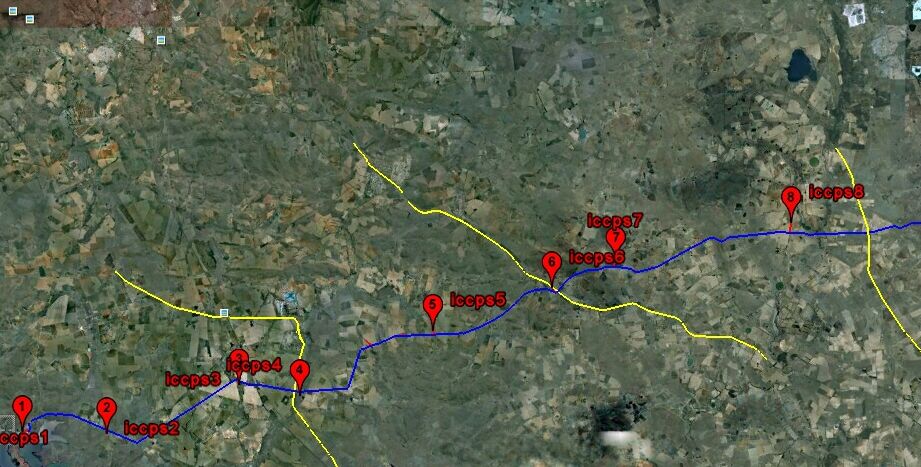
I was allowed to utilise some of my unique survey procedures to build an overview of the corrosion status of the region.
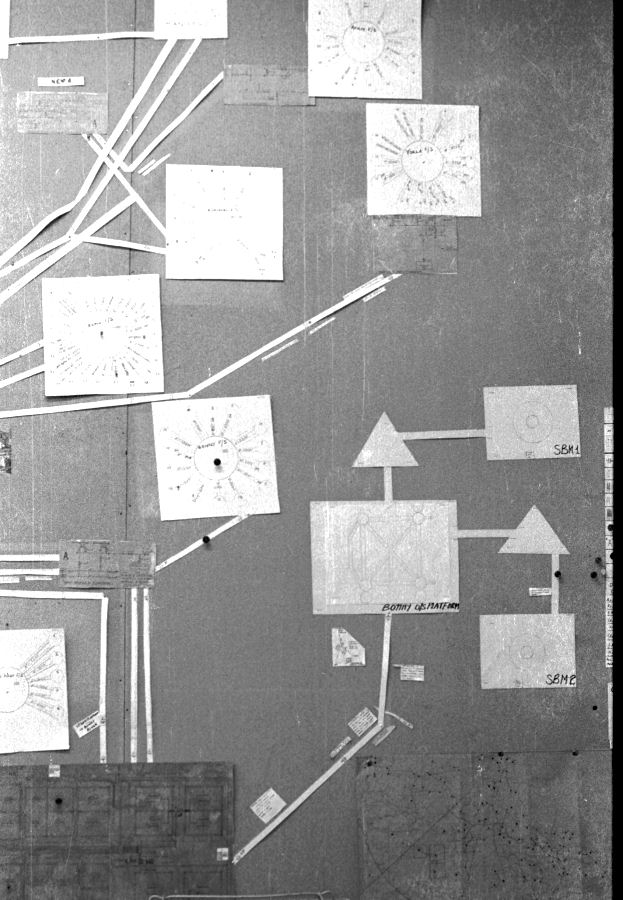
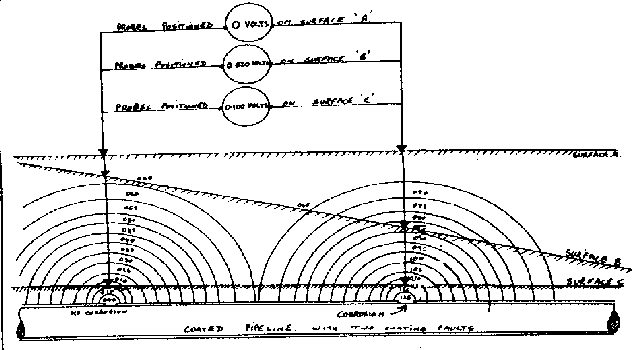
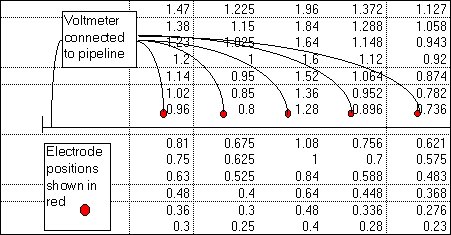
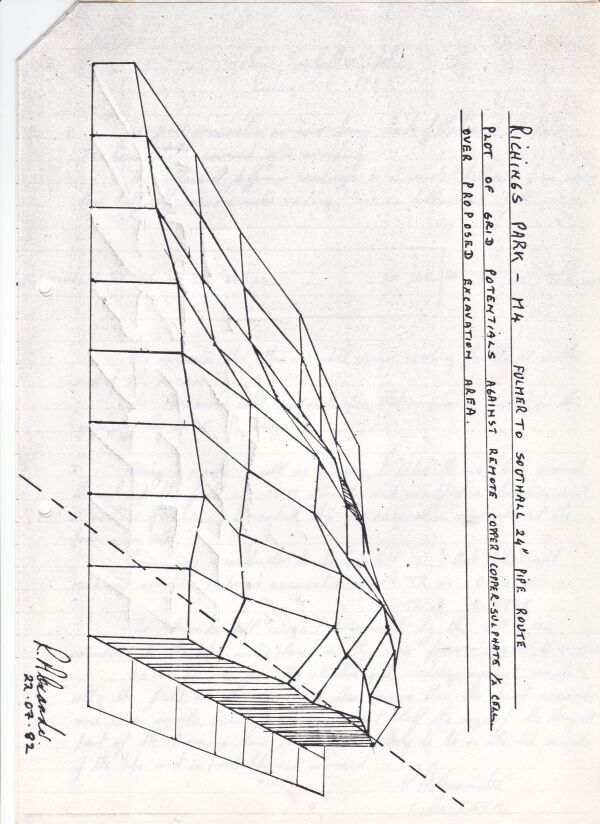
I specified the 'two half-cell' survey to a contractor Mark Derefaka in early 1975 at Bomu Manifold which had been bombed during the Biafran war.

Another manifold had been quickly built over the old one, to get the oil flowing, but there were no drawings of the old buried pipework as they had been lost in the destruction of some of the head office buildings.
This contract proved the value of mapping the potential profile of the ground and showed the exact position of all the old pipework prior to exacavation.

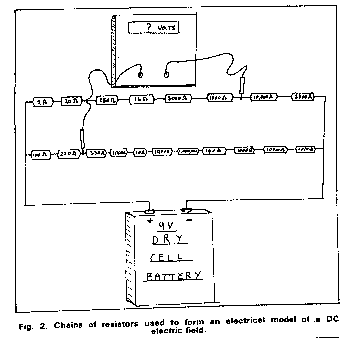
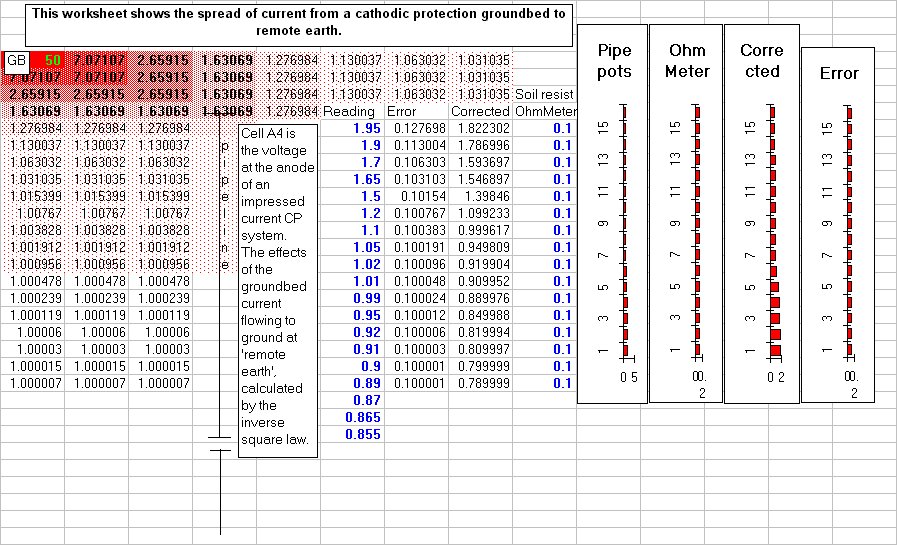
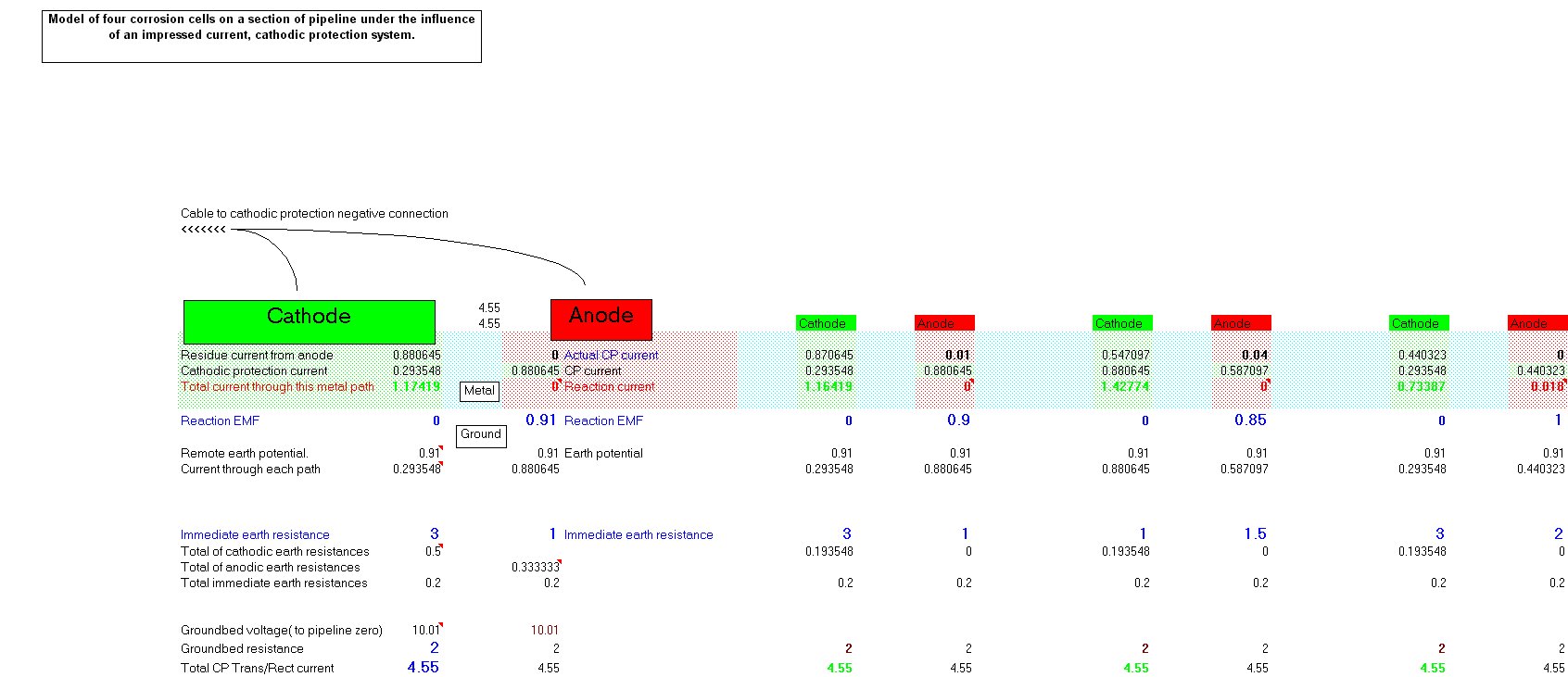
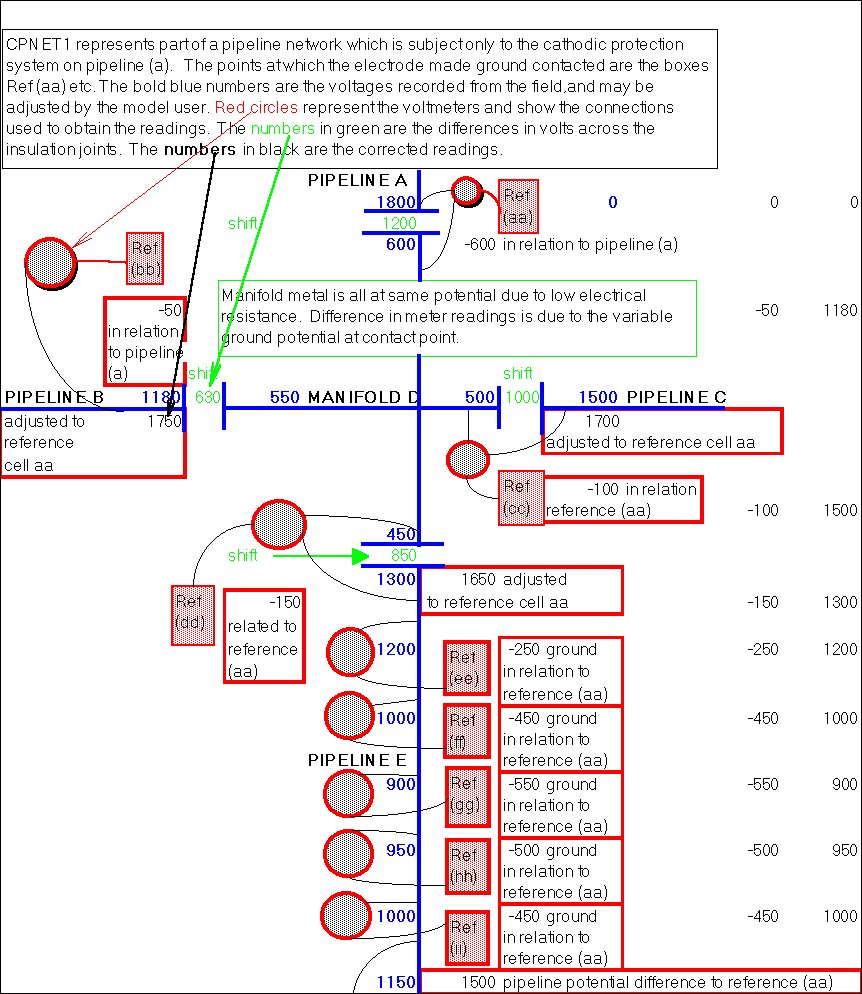
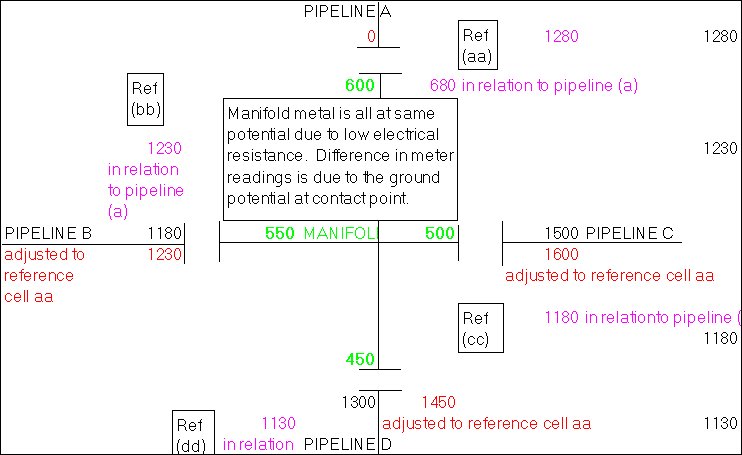
By regarding the whole network of pipelines as a massive electronic circuit, I was able to draw an equivalent circuit with impressed current systems and sacrificial anodes.


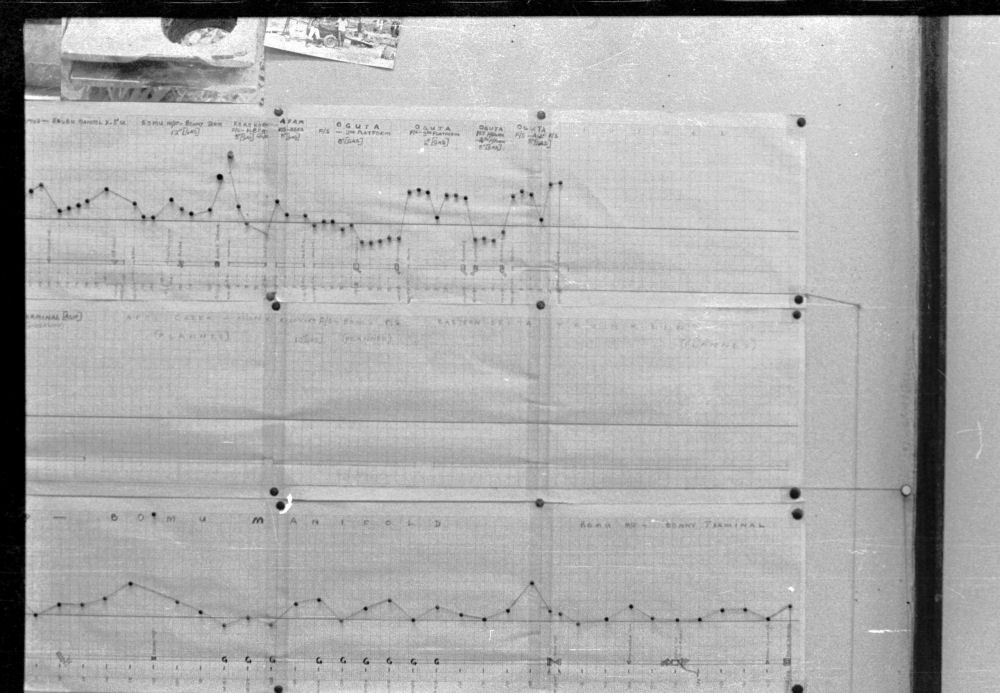

Pocket calculators had just become available so I was able work out the likely locations of corrosion using electrical laws and reconciling each part of the system.
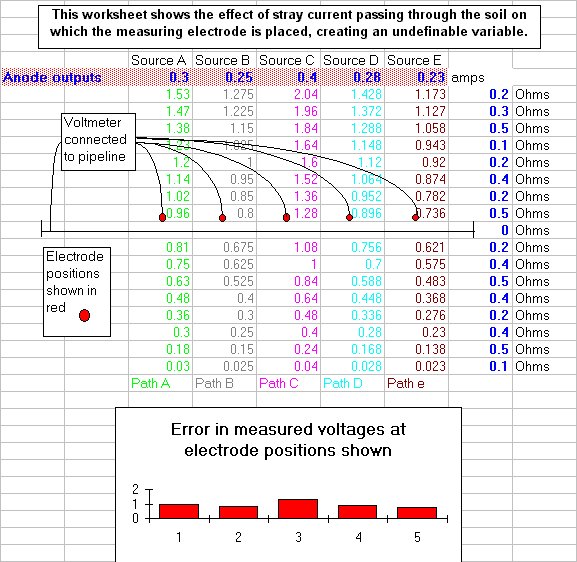
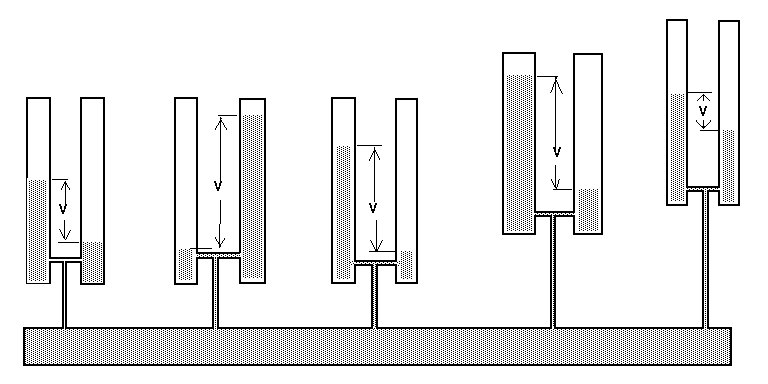
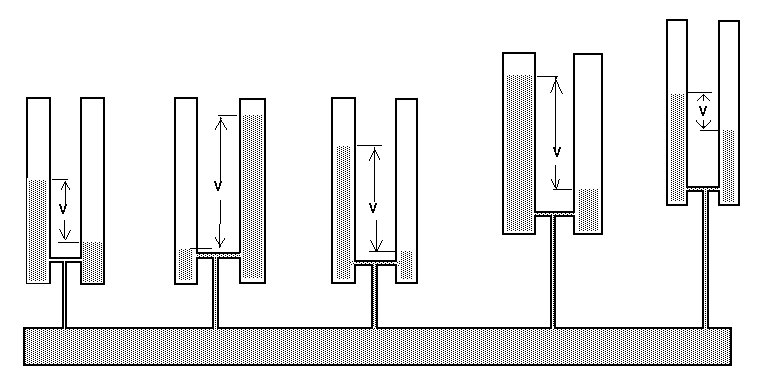
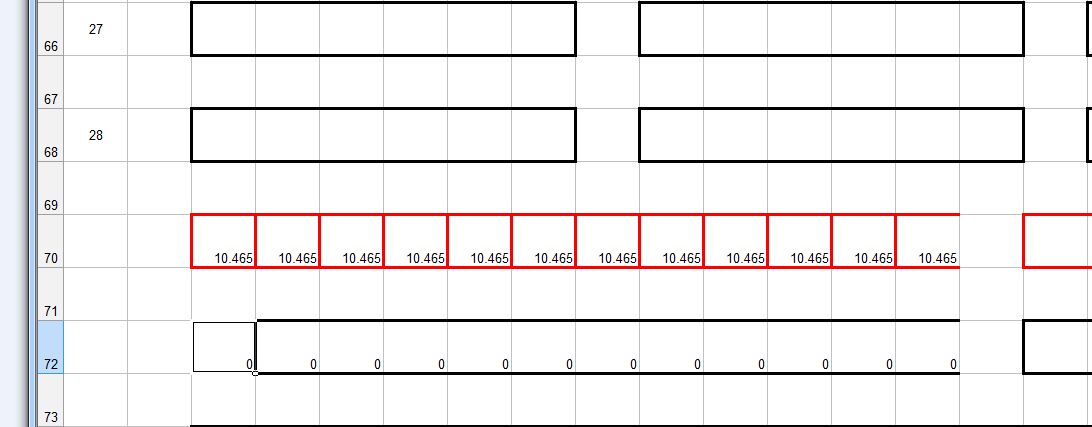
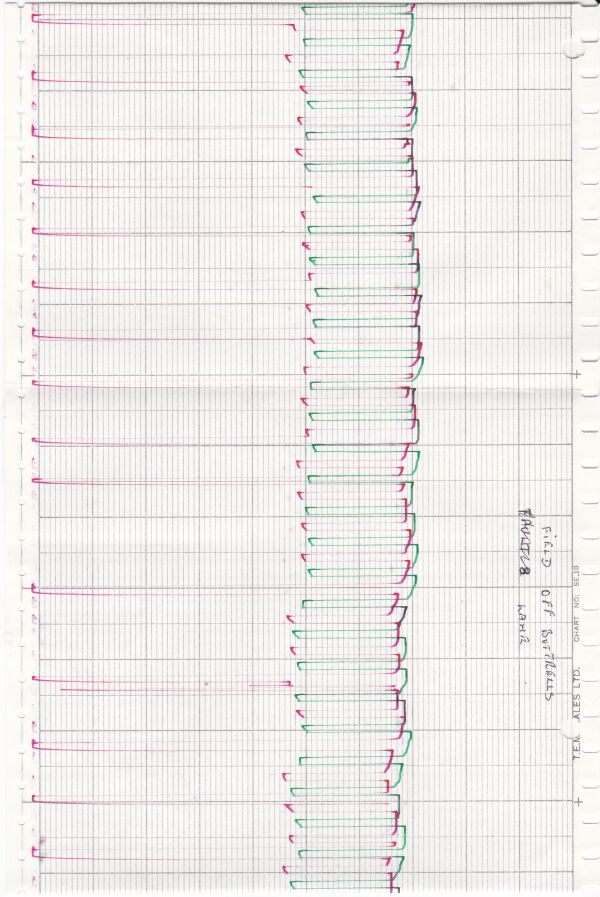
My survey teams would enter their readings on wall graphs using map pins linked with cotton.

Direct Current readings were shown on cardboard strips on a wall mounted schematic of the pipeline layout of the whole region.

The picture above shows a very small section of the layout.
By comparing data from the files with present field data I was able to predict, with a fair degree of accuracy, the likely locations of corrosion failure in the immediate future.
On one occasion Steve Mayaki returned from an investigative survey having found that a predicted location had actually started to leak. This was immediately remedied with a leak clamp having lost only a few gallons of oil and virtually no environmental damage.
I was able to bring the whole corrosion crisis under control in a couple of years and reduce the incidence of external corrosion leaks to zero in four years.
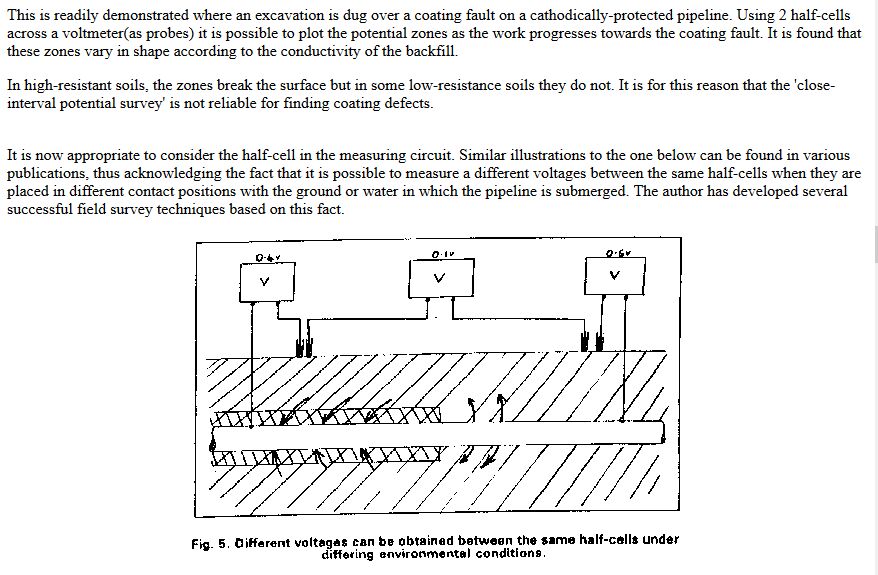
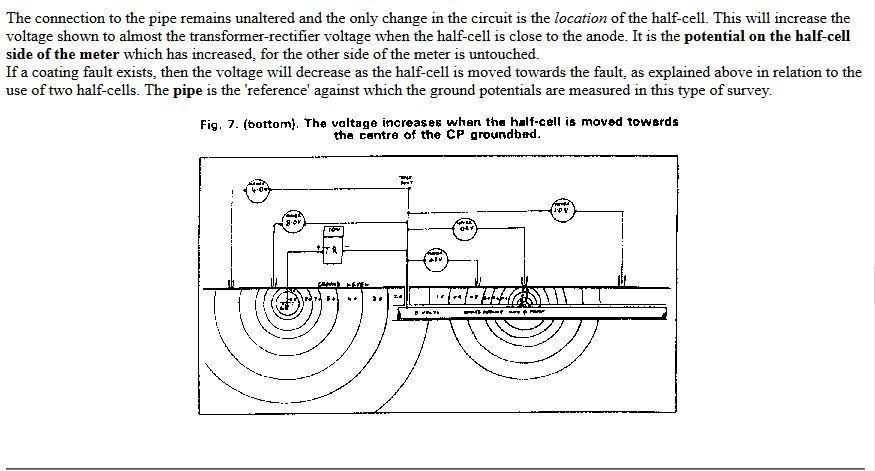
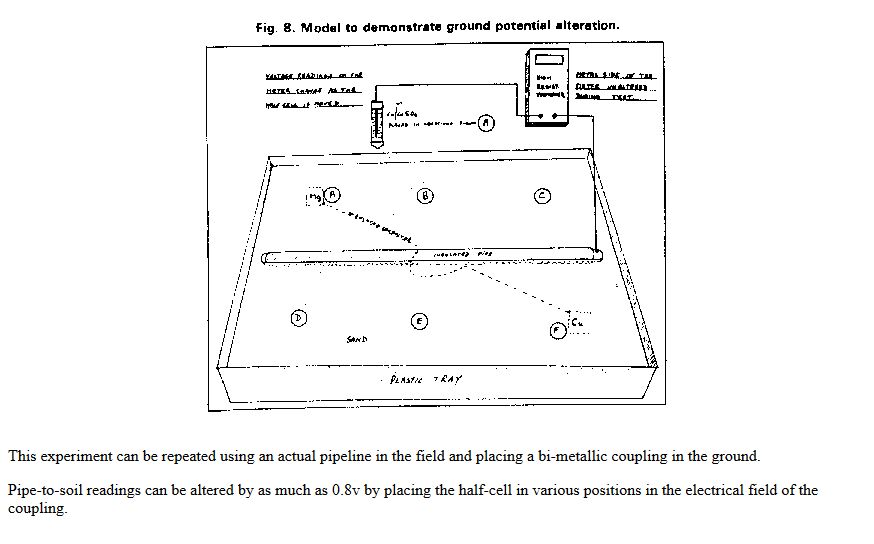
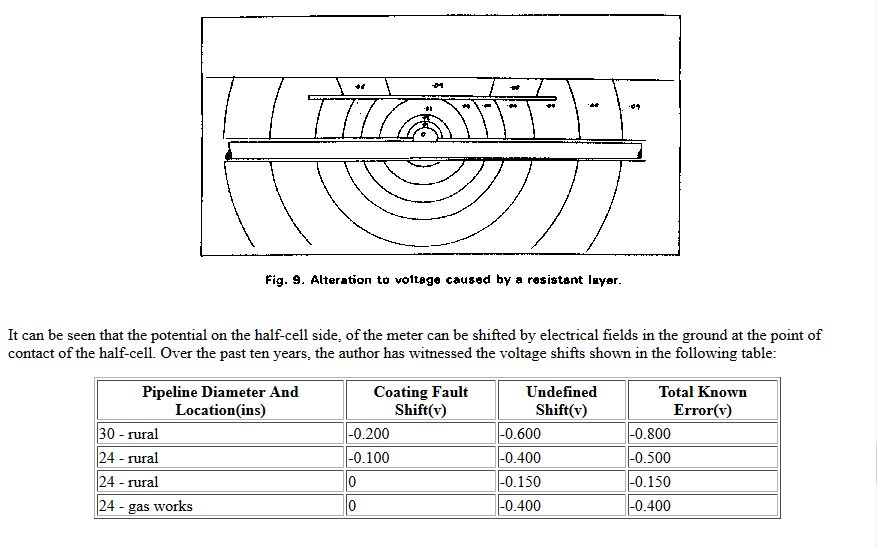
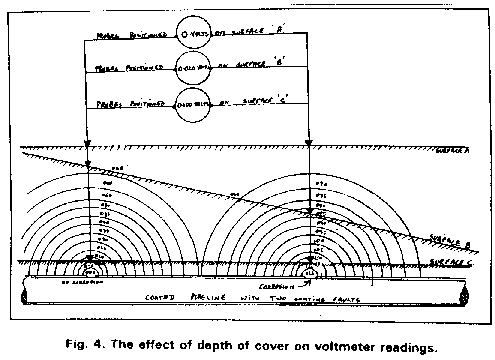
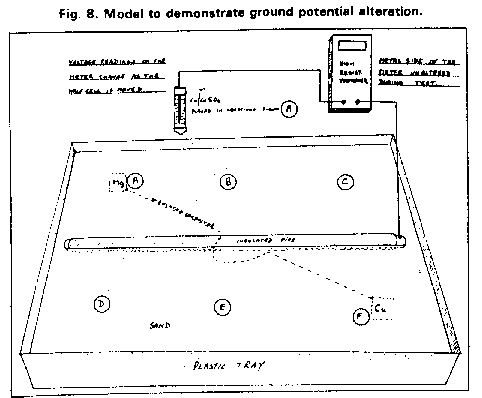
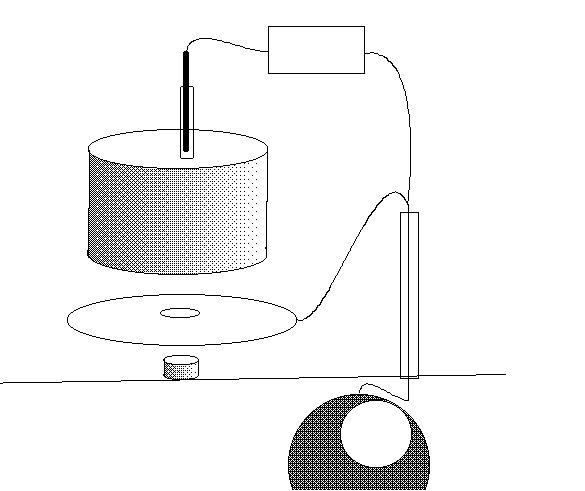
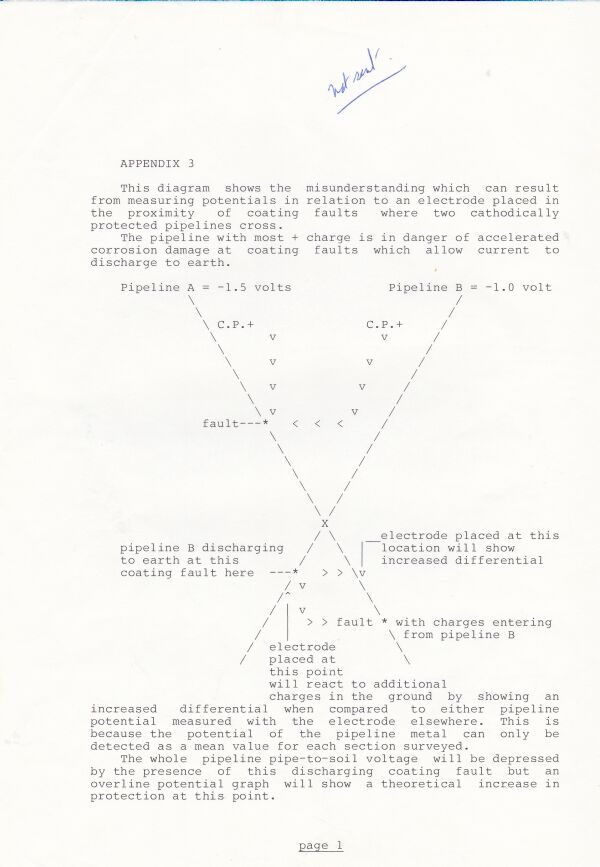
This extensive period of investigation and prevention of corrosion confirmed that there is no way that the 'half-cell' can be used to establish a reference potential but that it is very useful as a probe to contact the electrolyte in which the pipeline or structure is burried or submerged.
If a bare metal contact is made then that metal reacts to the salts disolved in the electrolyte, thus adding yet another EMF to the measuring circuit.
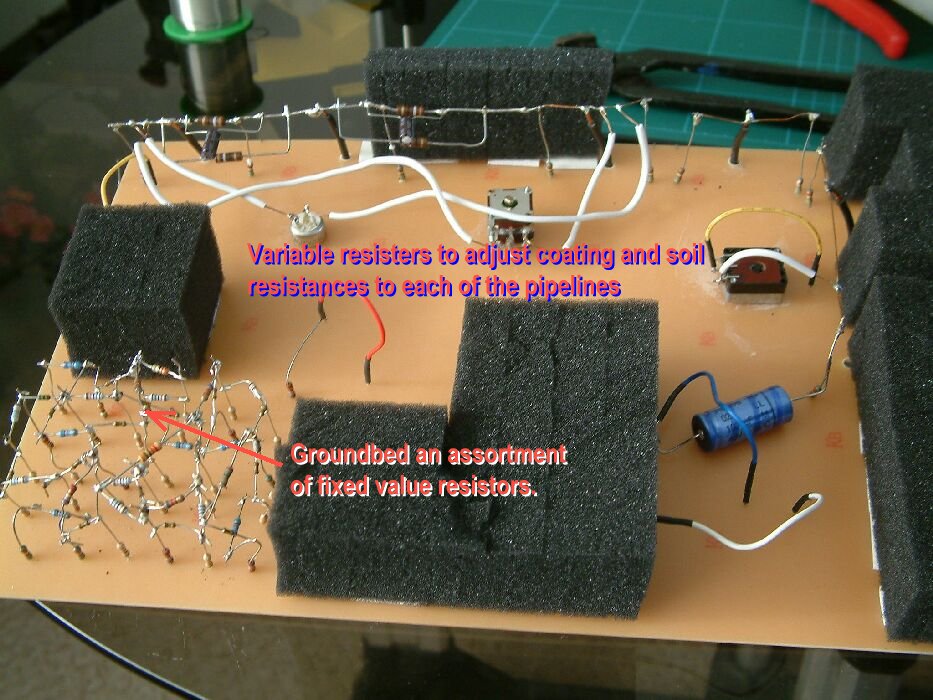
In 1978 I realised that the only way forward was to try to devise a method of measuring the actual corrosion current and the effect of cathodic protection on the corrosion reaction itself.
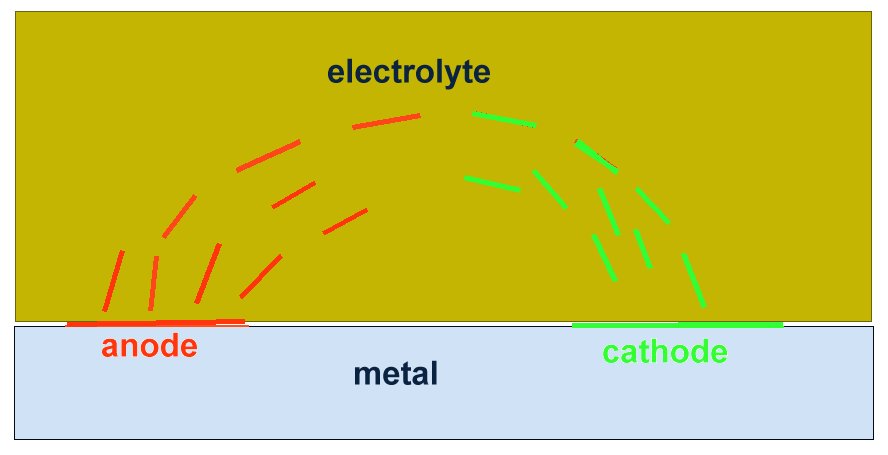
I used an assortment of metal coupons and sensitive meters but the problem was that the current must be measured without disturbing the reaction. The effect of the cathodic protection must be measured without disturbing the passage of that current onto either the anodic or cathodic interface.
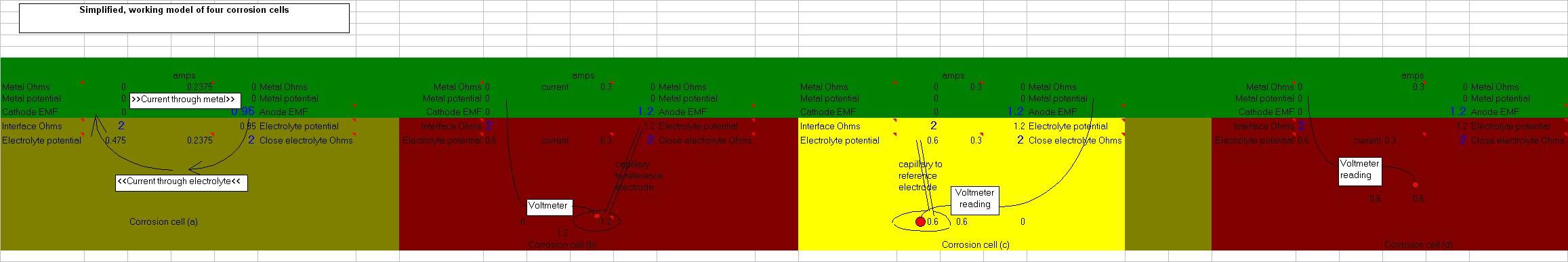
The only way to do this is to create a real corrosion cell in such a way that the corrosion current can be observed in any state of equilibrium.
Return to the UK
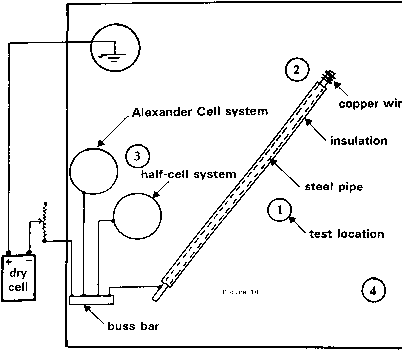
Family break up and single parenting forced a short period of independant research and development in the UK, during which time I designed the Alexander Cell into a working unit.
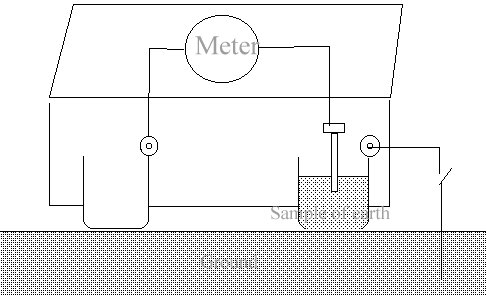
I had a solicitor sign this drawing to establish a priority date for my invention'
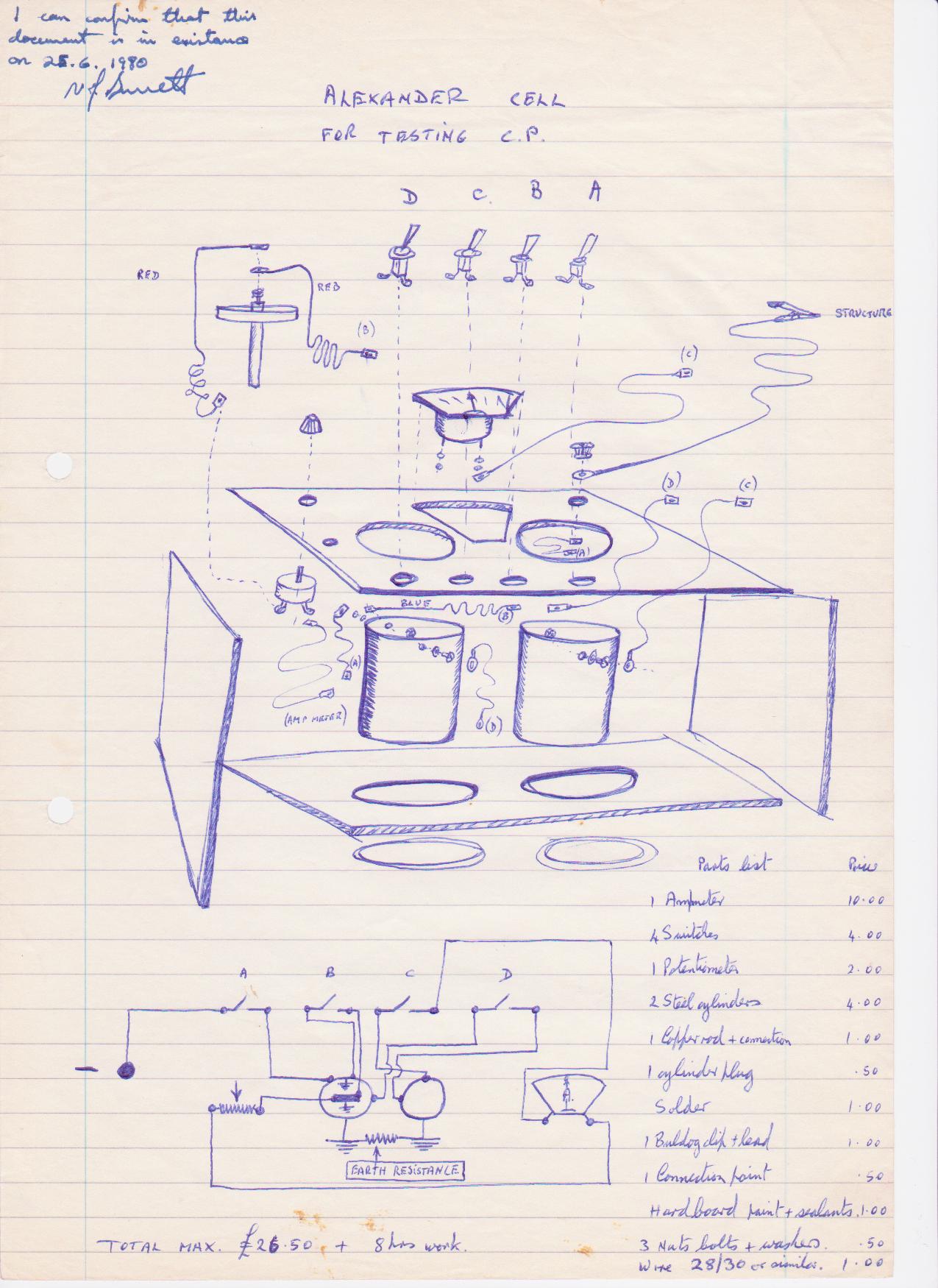
I then spent four years as a cathodic protection technician working first for Atkins Inspection and later with Global Cathodic Protection on contract to North Thames Gas.

Before I took the position I was interviewed by Mike Foskett, Chief Corrosion Engineer for North Thames Gas who was based at Staines. We discussed my experience in Nigeria and he agreed that I could conduct field trials of the Alexander Cell (in my own time) at North Thames Gas pipeline locations.
The project was to conduct a condition audit of many thousands of miles of high pressure gas pipelines delivering North Sea Gas to the north London area.
A mainframe computer had just been installed in Staines Head Office and the project was guided by Bob Greenwood of the Gas Council ERS.
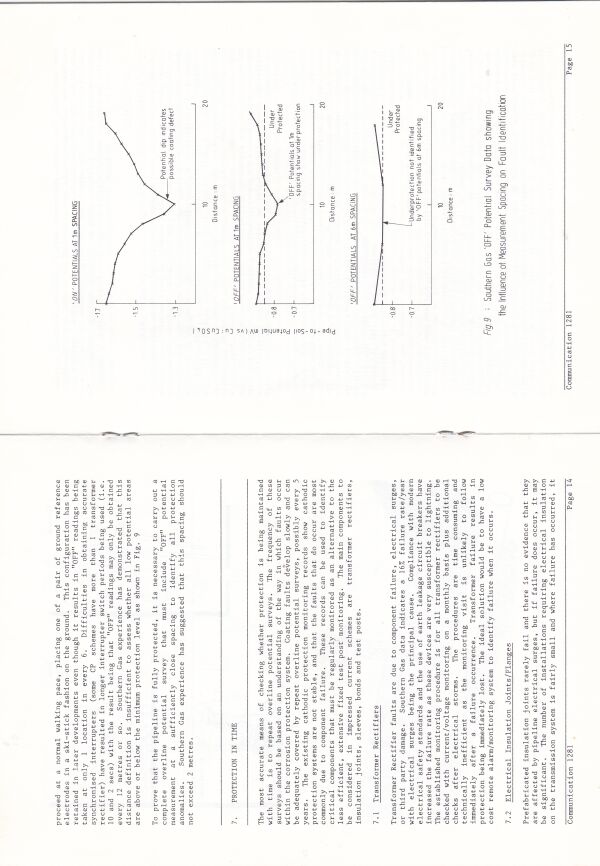
The procedure being developed was known as OLI1 (Over Line Inspection) and was developed in stages to OLI4 which is now known internationally as CIPS (Close Interval Potential Survey)
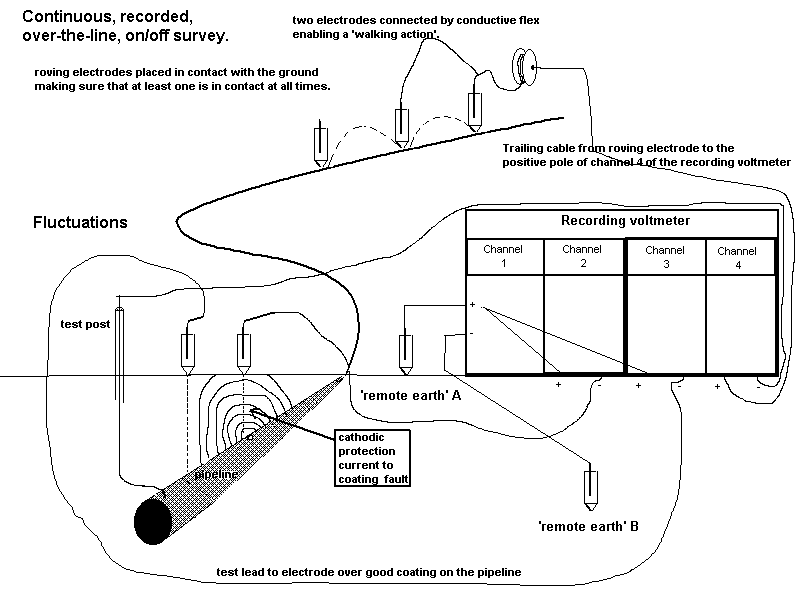
The reason for its development was the corrosion failures of pipelines that had been operated in compliance with the British Standards Institute Code Of Practice (CP1021) on which the pipeline licencing in the UK was based.
I joined one of four teams of technicians who were making notes of each voltage as the cathodic protection current was switched off and on at the nearest transformer rectifier.
Mike Fosket told me that they used the computer to plot the voltages in both states and that they had started by plotting the difference until they realised that this plot did not show the coating faults as they had hoped.
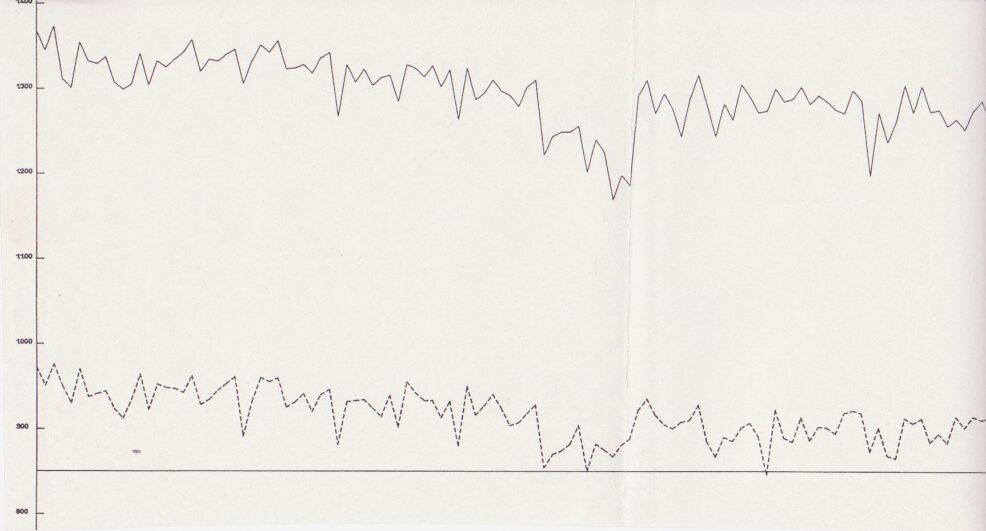
The theory had been that they could use the difference between the on and off readings to work out if the corrosion had been stopped.
In fact, they had found the principles that I had used several years before of plotting the ground potential profile.
I described the 'two half-cell' procedure that I used extensively in Nigeria and it was adopted as and additional check to locate the exact position of coating faults before excavation.
I was then required to carry out the final overline procedures, including the Alexander Cell, to produce a written report for each location before excavation.
The use of OLI4 procedures alone produced 7% accuracy and the complete Alexander Technology procedures produced 97% accuracy. The sampling was 100 excavations that I attended personally.
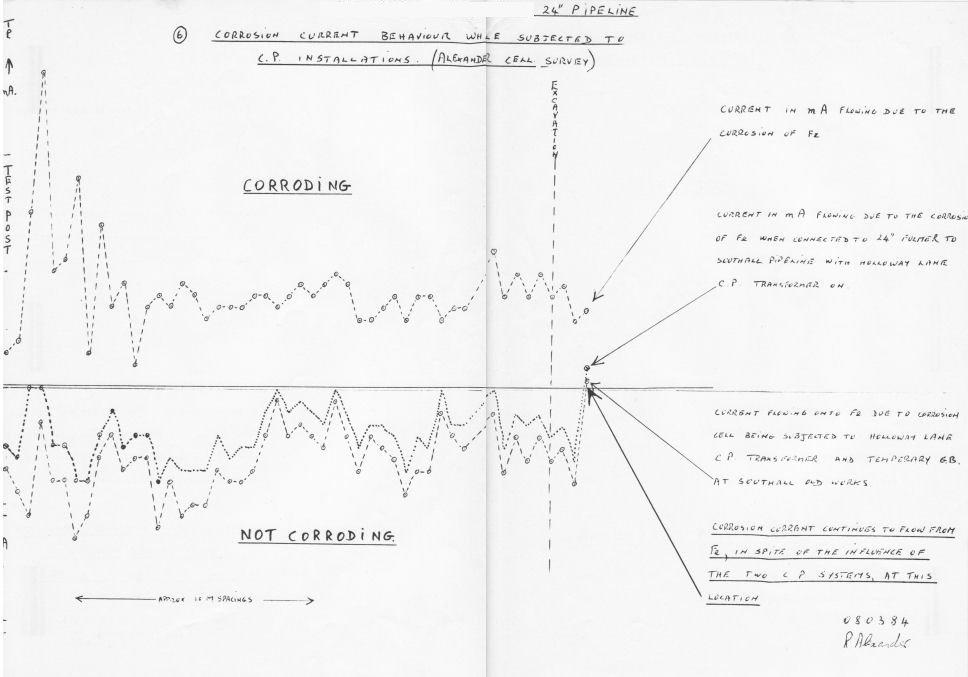
My success was such that Bob Greenwood visited site and saw the my procedures in action. He later authorised the purchase of an Alexander Cell, which prompted the Corrosion Engineer from South East Gas to buy one.

Mike Fosket asked me if I would like to write a paper about my view of cathodic protection which was radically different from mainsteam science at the time.





The paper was sent for technical editing to Dr Vic Ashworth of Ashton University, who rejected it completely with the comments that it did not fall within the concepts of known science.
This is the same Dr Vic Ashworth that used the words 'Cathodic shielding' for a situation where a highly resistant coating prevents cathodic protection current from reaching the metal of the pipeline. This is totally incorrect and has completely misled the coatings industry into manufacturing coatings that are not electrically insulating. This makes it impossible to spread impressed cathodic protection current. It is ignorant of real science.
Because of the success of my work in the field, Mike Foskette arranged for me to make a presentation to the London branch of the Institute of Corrosion Science and Technology.
This was attended by over 100 qualified, practicing corrosion engineers, over 60 signed the document confirming that they had been present at my presentation.



The Chairman of the BSI Committee for CP1021 attended and addressed the meeting after the presentation. He said that he supported everything he had heard and as a result was withdrawing the BSI Code of Practice 1021 for review.


The presentation included drawings, demonstration models and videos of field work to support the content of the talk.




I sent the video that I made on site and used at the presentation to the Open University hoping that I could somehow be considered as an expert that they could engage on a professional basis. Big mistake as a prominant person in the Open University was Bryan Wyatt, who was the CEO of Global Cathodic Protection Ltd of Telford, and who wanted to buy the Alexander Cell so that he suppress it.






































































1983-1984 Global Cathodic Protection Ltd. continuing the same work for North Thames Gas.





1984-1987 Alexander Corrosion Technology Ltd, CEO marketing the Alexnder Cell, cathodic protection monitoring and a corrosion control service to industry. Our work was to be in Libya where our Italian financier had friends and owned companies but a political situation caused cash flow problems.

1985 may


1986

1986 sept

1986 oct





1986 nov


 History of Roger Alexander
History of Roger Alexander
1944-1949 Church of England Primary Education
1949-1955 Eastbourne Grammar School
1955-1957 Boots the Chemists, apprentice Pharmacist.
1957-1960 H.M. Forces, UK, Germany and Malaya.
1960-1965 Eastbourne Borough Police Force
1965 -1968 William Press and Sons, Industrial Safety Officer. Overseeing site safety during construction and service to the oil and gas industry.
1968-1972 Laing Pipelines, Chief Safety Officer, responsible for all safety aspects of natural gas pipeline work, including inshore pipeline construction.
1972-1973 Mohammed Salem Al Suwaidi, Construction Company, Saudi Arabia, Safety Officer for the construction of the pipe work at ARAMCO Ras Tanura export facility.
1973-1973 Mapel, Coat and Wrap inspection and training as a Cathodic Protection Technician
1973-1974 Mapel-Ghar Ltd Iran, supervising constructing and commissioning cathodic protection facilities and trouble shooting
1974-1978 Solus Schall, contract staff to Shell-BP Development Company Ltd of Nigeria.
Based at Port Harcourt, my title was ENGE18, Head of the Corrosion Engineering Section for the Eastern Division.
1978-1980 Family break up caused me to be single parent to my 10 year old son and work from home as a freelance photographer.
1980-1983 Atkins Inspection Services Ltd Cathodic Protection Technician engaged in field work for North Thames Gas based Slough.
1983-1984 Global Cathodic Protection Ltd. continuing the same work for North Thames Gas.
1984-1987 Alexander Corrosion Technology Ltd, CEO marketing the Alexnder Cell, cathodic protection monitoring and a corrosion control service to industry. Our work was to be in Libya where our Italian financier had friends and owned companies but a political situation caused cash flow problems.
1987-1991 Consultance work relating to the application of computer technology to cathodic protection analysis.
1991-1993
1993-1994 Nigeria, Thames Valley Berkshire Business Growth Hub.

2008-2009
2007-2009
2009-2010 Brazil, building training centre at Guararema, SP.
2010-2011 South Africa, UK
2011-2012 Iran, UK
2012-2013 Germany, UK
2013-2014 Brazil, UK
2014-2015 Iran, UK
2015-2016 Brazil, UK
On Tuesday 9th February 2016 I went to the drop in clinic at Bracknell Innovation & Enterprise Hub where I met Chris Farrance. I described the progress I have made since meeting Susan Elliott many years ago and explained that I do not want money but need assistance to continue to expand my company that I registered for the purpose of giving away my intellectual property with respect to the control of corrosion on networks of steel pipelines. He looked at my web page
Susan Elliott
I explained that The control of corrosion required research and development at university level, software development, training, manufacture, construction and installation engineering, commissioning and maintenace activities that must be coordinated and synchronised. He asked for a business plan and I explained that there were business plans for each of these activities included in the information that I had already given to Susan Elliott.
I have uploaded these for continuity, for the convenience of anyone interested in helping me.
Chris told me that he could not help and I asked him to refer the matter to someone who could.
2016-2017 Brazil, UK
2017-2018 Brazil, UK
2018-2019 Brazil, UK
2019-2020 Brazil, UK






















