Three day training seminar
Period 9
Actions
We have seen in the first day of this seminar that the application of cathodic protection is problematic as it cannot be deterministic like other engineering practices utilising electronics.
During period 1 we saw that Orac 2 represents the measurements of corrosion cells typical of the arrangement that we face every day in cathodic protection field work. These measurements cannt be resolved.
We saw that electrical measurements can be made by contacting two locations in the electrolyte and that this is the process used when I invented DCVG in the 1970's.
We saw that 'pipe-to-soil potentials' are in fact voltages between two floating potentials in a measuring circuit thatthat cannot be resolved.
We saw that the Alexander Cell is simply a corrosion cell in which the anode is separated from the cathode allowing a measurement to be made in compliance with DIN50918 and other laboratory techniques.
I explained that these matters had been discussed at the highest level in the UK and that published papers had been read and approved globally during the 1980's.
During period 2 we examined DIN50918 and the copper/copper-sulphate electrode that is NOT a 'half-cell' but is a known reaction potential that can be compared to another reaction potential in closed circuit condition.
We saw that this closed circuit condition can only be achieved in field work using the Alexander Cell.
We saw that all other methods of field measurements include more than two variables that cannot be resolved and that physical demonstrations are necessary to prove this to people who have been trained to believe otherwise.
We now know that the present methods of making measurements in cathodic protection field work do not result in data that can be analysed by computer circuit analysis.
During period 3 we discussed the issue of the measurement and the attempts that have been made over many decades to define a criterion or measurement at which we can be certain that we have controlled corrosion.
I described instrumentation that was proposed and field trialled to overcome this recognised problem.
I described the attempts to measure the 'polarised potential' of subject structures using the immediate off voltage measurement, and how that is NOT what the scientists need to establish a base line for the equations that they have codified.
I described how the Alexander Cell can be used realistically with the CP systems in the 'on' mode, and that this is the mode in which they are designed to operate.
During period 4 we examined how instrumentation works and exactly what it measures.
It was made clear that zero is set each time we make a measurement and this is relative to the common input of the meter that is in use.
This clarifies the actual measurements that we represnt on the graphs that are commonly used in cathodic protection work. They are upside down by camprison to graphs used in conventional electronics, and this is unnecessarily confusing.
During period 5 I described in detail the physical equivalent circuit called Orac that was made to test the suggested equivalent circuits published in most authoritative publications relating to cathodic protection.
During period 6 I described the evolving physical model of cathodic protection that is named Orac 2 and how it has now developed into a full laboratory that is capable of simulating all of the features of a complete cathodic protection network of pipelines and facilities.
Period 6 ended with the proosal that it is now possible to organise and build a central control for all corrosion on networks of pipelines and assets.
Period 7 stressed that in order to achieve central control we need a criterion that can act as a zero for all our calculations over the integrated circuit of the global cathodic protection network.
A cathodic protection system is an electrical circuit
In the same way that it impossible to design a computer or mobile phone without a circuit diagram that shows the corrosion cells (batteries and power supplies) that balance the equilibrium to make these circuits function.
Equivalent circuit drawn in 1977
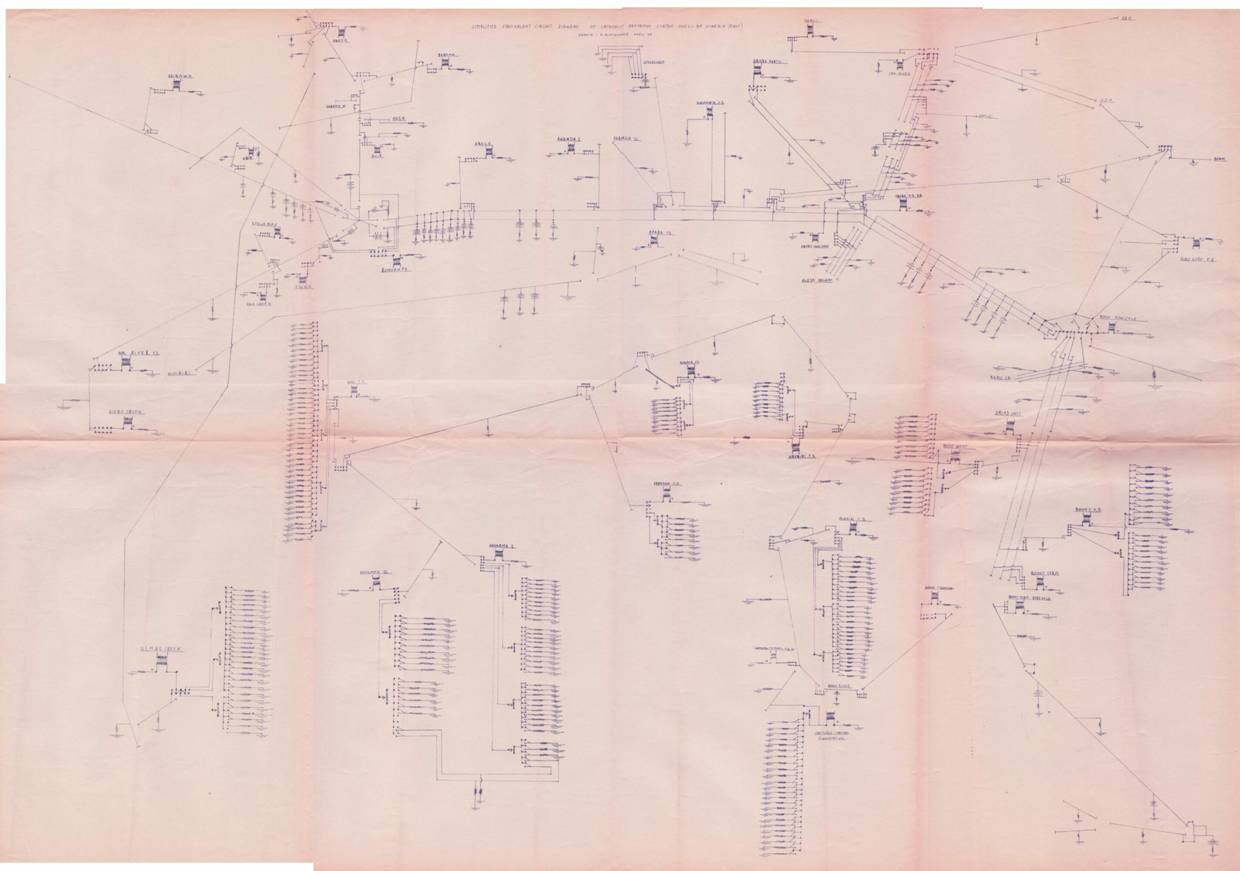
Co-ordination required.
It is impossible to control corrosion to networks of pipelines without co-ordination and synchronisation of activities. It is impossible to control corrosion within a tank farm without an equivalent circuit diagram that can be used to balance the electrical equilibrium of the whole area.
This is an integrated circuit
Every adjustment alters the equilibrium of the whole.
.
Cathodic protection network is a physical circuit composed of all the metallic paths that cover the globe.
This is a demonstrable scientific and technical fact.
Until the internet came into being it was impossible to control corrosion on networks of pipelines but now it is not only possible but very desirable and economically imperative.
Global co-operation
We need an international convention to codify these activities based on present experiences and results.
We must therefore use the internet to gather all the experiences and specialised knowledge for discussion without constraints.
It must be free to all users and open to all contributors.
It must be based on repeatedly demonstrable observations in support of each element of codification.
It must be translated into all languages and understandable by all of those who practice in the field.
The rules of nature govern the universe
We need academia and government supported research and development or 'competition' will result in restricting the flow of information.
Pipelines cross man-made and natural borders but the same rules of nature MUST BE OBEYED.
Cathodic Protection Network has achieved much in this respect but I now suggest that the name of all our activities be changed to something more easily recognised such as Electronic Corrosion Control
The activities will be
Consultancy.
Software development.
Training.
Manufacture.
Human resources.
Installation, commissioning and maintenance.





