Three day training seminar
Period 8
Cathodic Protection Criteria
At present we tell clients that a graph tells us when we have achieved cathodic protection of a pipeline.
This graph displays voltages joined together incorrectly and we say that the topmost trace represents the pipe-to-soil potential with the cathodic protection system switched on.
We say that the lower trace represents the 'polarised potential' measured immediately after all of the impressed current cathodic protection systems have been switched off.
We do not remove the errors in the voltage reading by switching the power sources off but we do change the normal equilibrium of the system that is designed to control corrosion.
The measurement that we make is a voltage made during a slope in the graph produced by the sampling system of the instrument and how the computer is set to chose an average of the range of voltages.
The original specification for the periods on and off was 12 seconds on and 3 seconds off but the 'off' readings could sometimes not reach the require criterion so they have been changing the recommendations ever since.
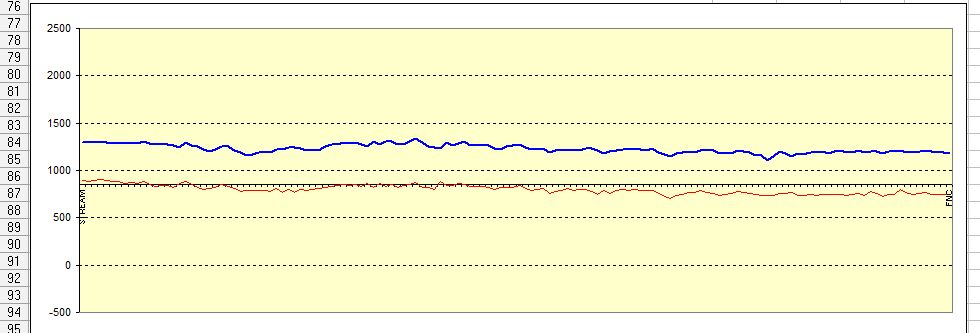
There are some companies that are now using milliseconds off and all they are producing is a saw tooth shaped graph that is displayed by the computer as on and off readings separately.
This has degraded the CIPS survey into nonsense, as shown clearly in Period 3 of this seminar.
The ultimate criterion
The ultimate criterion for the success of cathodic protection is the continuing operation or failure of the pipeline.
Of course we do not have to wait for a failure to occur as we can excavate and make a physical examination of the pipeline.
Such examinations have been enhanced by the development of the 'intelligent pig' but these methods do not indicate the measures that must be taken to stop the progress of any damage that is found.
The next most certain criterion is to install a series of 'weight loss coupons' which will reflect the performance of the pipeline metal, but this is also long term exercise and has practical difficulties. It requires expert and highly trained technicians to apply the system and is not commonly practiced.
Cathodic protection is based on the fact that corrosion is an electro-chemical process and the electrical element can be controlled and measured.
It would seem that a simple electrical measurement would suffice for a criterion which would indicate when corrosion has stopped. After all Faraday determined that the amount of metal that goes into solution in the corrosion reaction is directly proportional to the amount of DC current which results from that reaction.

The problems in making field measurements are discussed elsewhere but until the early 1970's it was generally believed that a standard reference electrode could be used in cathodic protection field work and the instructions were that it should be placed as close as possible to the pipeline or structure.
The many criteria
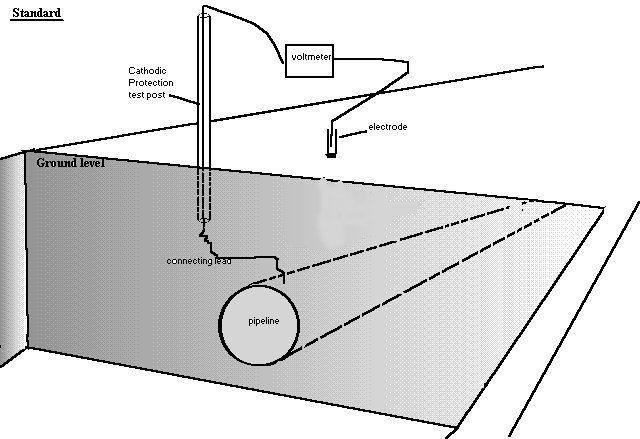
It was believed that if the pipeline became negative to a copper/copper-sulphate reference electrode in excess of 0.850 volts, then corrosion had been halted. The academics supported this notion and theories had been established which fitted in with thermo-dynamics.
The 'Pourbaix' diagram was published and hailed as the proof that the -0.850v criterion in relation to a copper/copper sulphate electrode was valid.
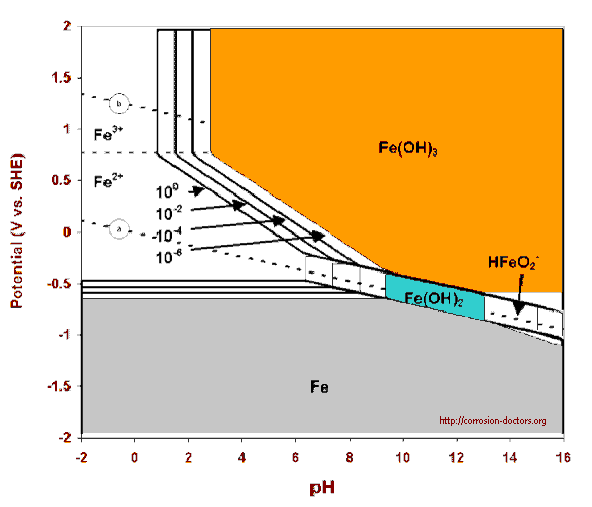
Evans produced more diagrams and the scientific evidence tended to support the empirical value that seems to have developed in field work.
However, pipelines have continued to leak, even when this criterion has been reached, and new criteria have been proposed, but none have been adopted internationally.
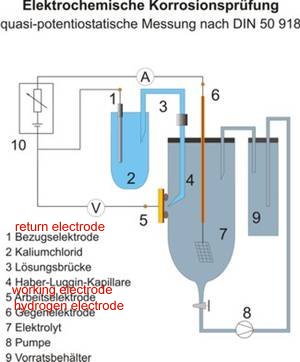
The only one definite criterion for cathodic protection is when corrosion has stopped and that is only measurable using the Alexander Cell.
Mainstream specialists are not pleased that this shows that the old criteria are sometimes as far as 300% in error.
I have in my possession seven scientific papers specifically on this subject and many others that mention the problems involved in establishing when corrosion has stopped.
Much research has been carried out, resulting in a number of techniques being suggested, and devices patented. None of these devices have achieved international acceptance and there is still no criterion for cathodic protection that is accepted by all mainstream specialists or their organisations.
The criterion needs to be easy to understand and the instruments required need to be rugged and practical for field use.
The papers that I have studied are complex and rely on the reader being conversant with details of corrosion science. It seems that these are not well understood by those that need to make the day to day decisions on pipeline maintenance and the application of the recommendations contained in the papers.
The real criterion
When corrosion has stopped we have achieved cathodic protection.
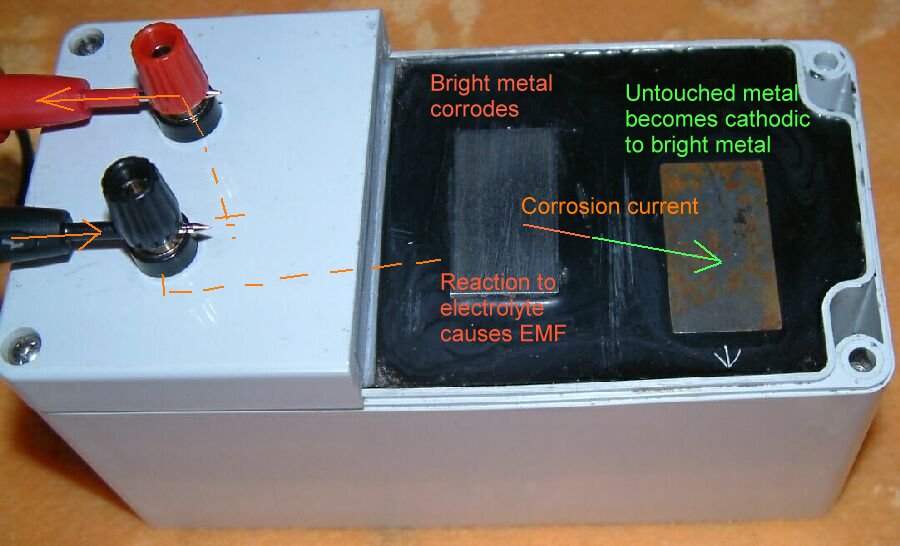
I presented a paper at two international conferences in 2009 in Brazil and these papers have not been challenged. It must be assumed that they are accepted.

The result

Training centre Guararema
Models

.
Actual

Training

Reality
The criterion can be tested at this training centre
















