
Cathodic Protection Training Course
Module 8
Resistivity effects on corrosion

The resistivity of the electrolyte has two affects on corrosion and corrosion control.
The first is to the electrochemical process that is widely studied in laboratories by scientists in the corrosion control and battery manufacture industry,
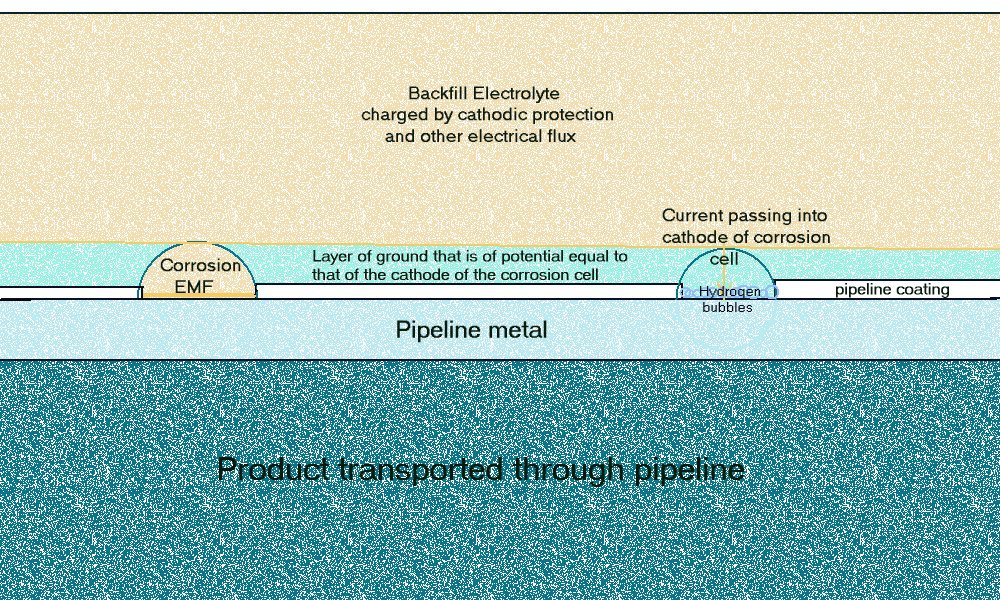
and the second is the effect of the ground resistances over larger distances where the passage of DC charges alter the ground potential.
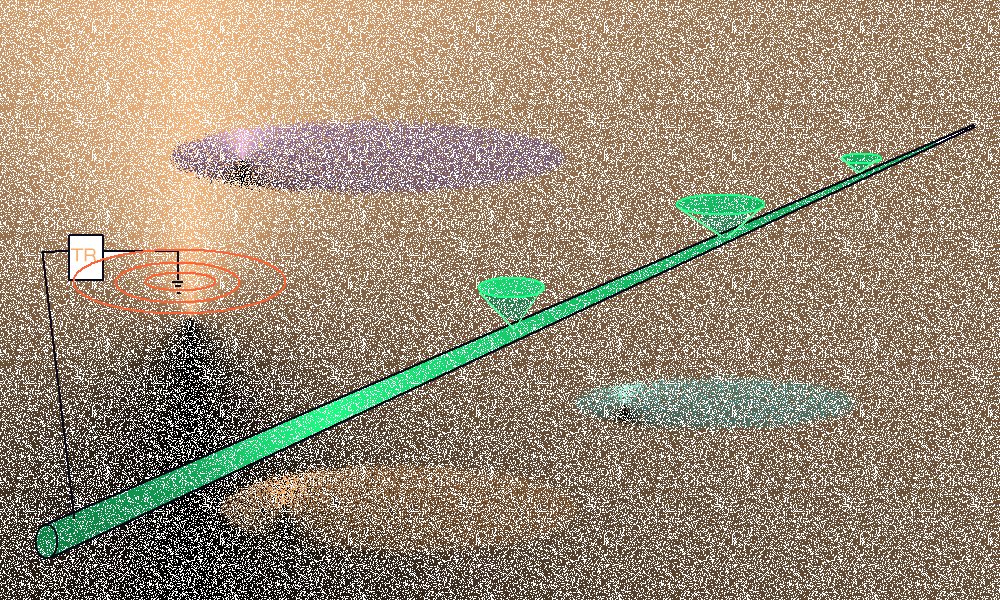
The pipeline metal has a very low comparative resistance but the variety of resistances in the 'open circuit' ground path is considerable.
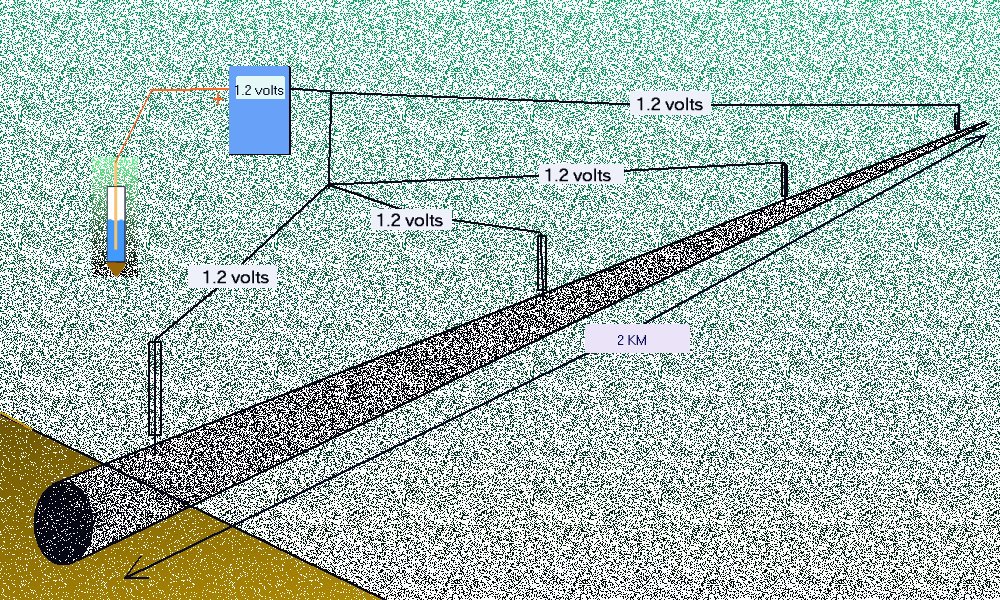
Pockets of low resistance ground are found over pipeline wayleaves and sometimes these result in 'long line' corrosion cells' where the anode can be some distance from the cathode.
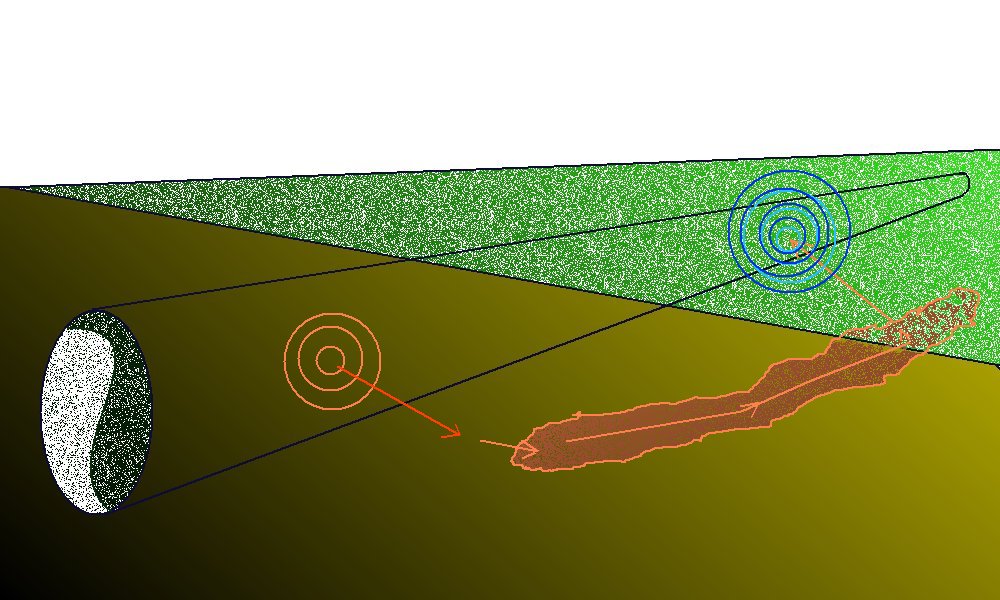
It is the resisivity of the backfill that has the most effect on corrosion cells on a pipeline. As cathodic protection engineers we must regard the coating as the first layer of backfill and any coating faults as locations where the second layer of backfill touches the pipeline metal.
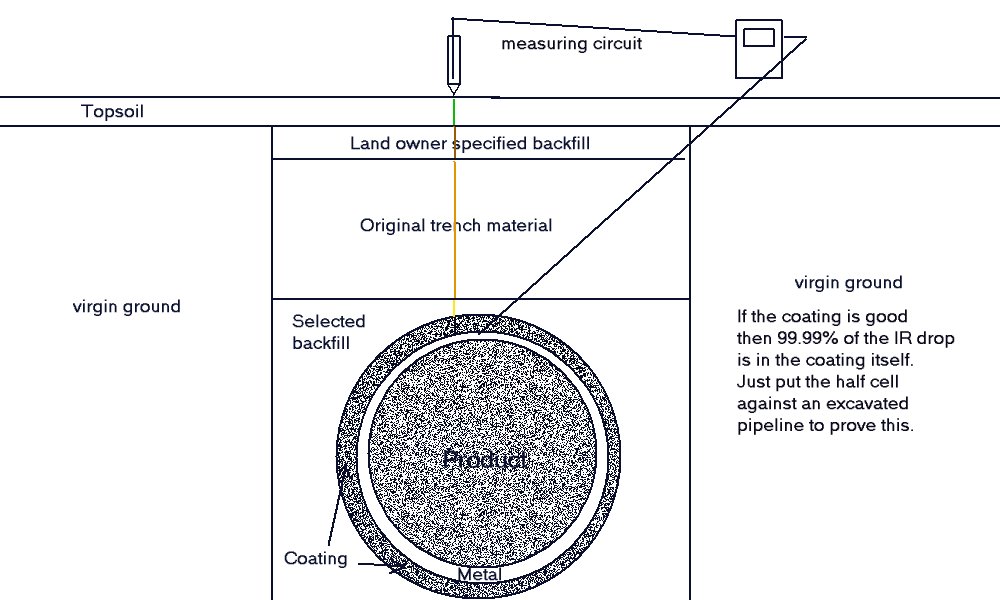
The potential difference across the coating is normally the same potential difference from the pipeline metal to 'remote earth'. This is because coatings are designed to be resistant to electrical charge transport as well as chemical degradation.
The next layer of backfill is selected to be electrcally resistant and chemically inert but of course gravity causes water in the area to utilise the trenct as a watercourse as soon as it is excavated and after it is backfilled.
This means that the belly fo the pipeline might be submerged in water with the disolved salts of it's origin. The belly of the pipeline is asol the most difficult to access for coating inspection and repair during construction.

Back to Module08 index page