
Cathodic Protection Training Course
Module 4
8 cathodic protection models

This is a demonstration to show real corrosion in less than three hours. In order to achieve this we must accelerate the reaction using the 'interference' of other corrosion cells, in a dry cell battery.
The dry cell battery consists of four corrosion cells arranged in series to produce 6 volts.

The nails are arranged on a super-absorbant cloth as shown above. This is covered with another cloth and soaked in water.
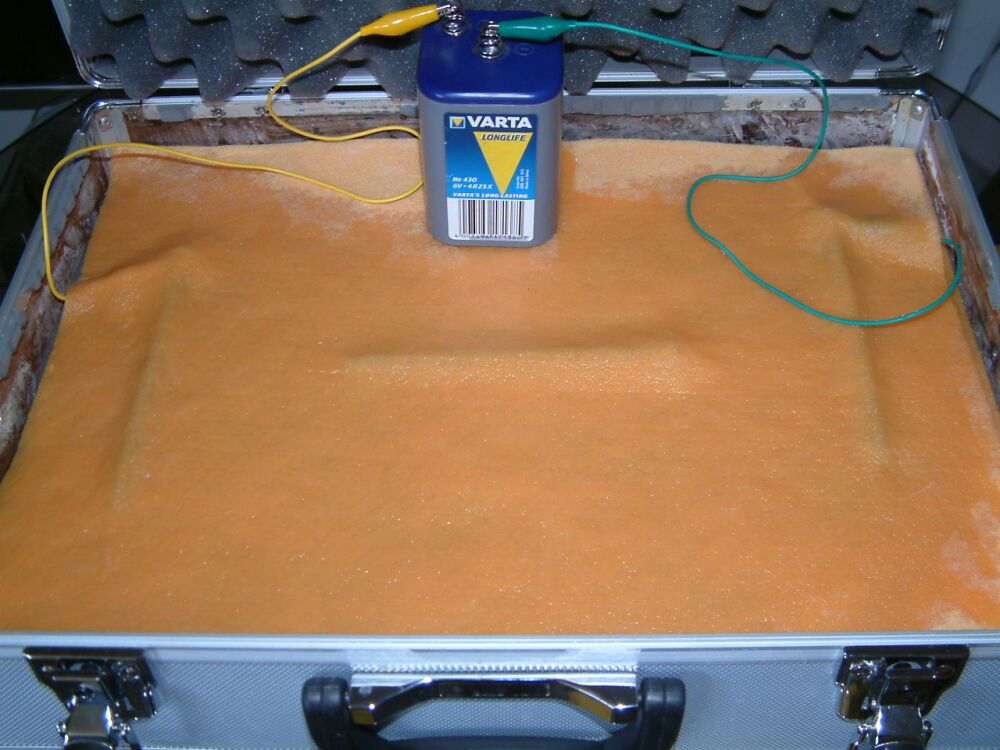
The nails are connected to a 6 volt battery as shown and the corrosion reaction starts where the current from the battery leaves the nail and emters the saturated cloth.

Using a digital multi-meter and two half-cells it is possible to 'see' the current paths that are accelerating the corrosion. It is possible to log these paths in such a way as to create a 'potential profile' of the whole cloth.

After three hours the covering cloth is removed to reveal the corrosion products and slight pitting where the metal has disolved.
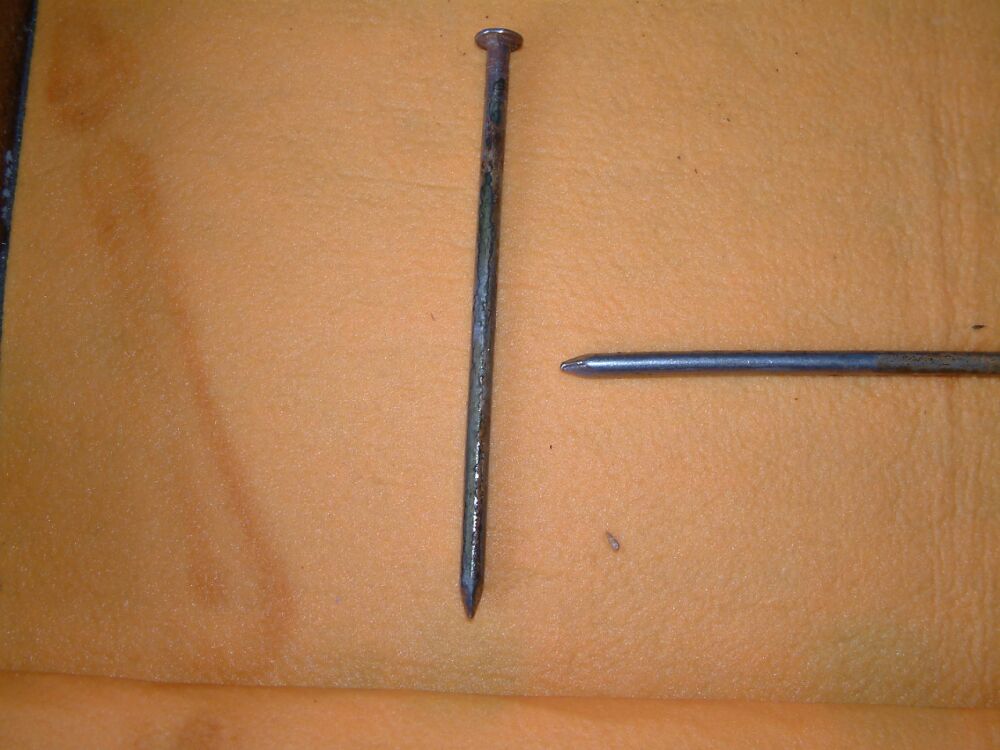
It is seen that the nail that was connected to the positive pole of the battery is corroded where it has been in contact with the saturated cloth. The nail to which the current has travelled is bright and shiny at the end closest to the source of the current and has been cathodically protected.

At the other end of the central nail is the effect of 'interference corrosion' where the current has left the metal and is protecting the nail onto which the current is passing. The bright area close to the head of the nail is where the cloth did not contact the metal. There was no electrical conductance here during the demonstration period.
This demonstration piece makes it possible to show the whole theory of cathodic protection in half a day. We can use this piece to experiment with many features found on site.
back to 8 Model index page