

|
Cathodic Protection Training Course
Module 3
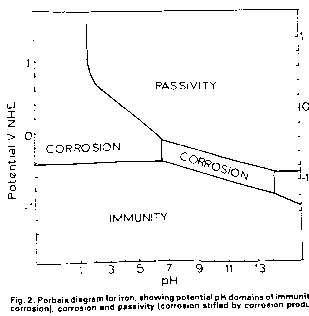
The significance of the Pourbaix diagrams.


|

People will refer to the Pourbaix Diagrams and scientific papers by Pourbaix and Evans because they are recognised as having explained how cathodic protecion works in thermo-dynamic terms. That is what I have been told by qualified scientists and I am not about to argue with them... they have been paid a lot more than me for their conclusions!
I have reproduced the Pourbaix Diagrams several times and believe that they show a state of equilibrium in which metal has not got the energy to disolve. It does not matter what equations etc he used to prove this as everyone believes him ... so that's OK.
It is VERY IMPORTANT to understand how the measurements were made to obtain the values included in these equations.
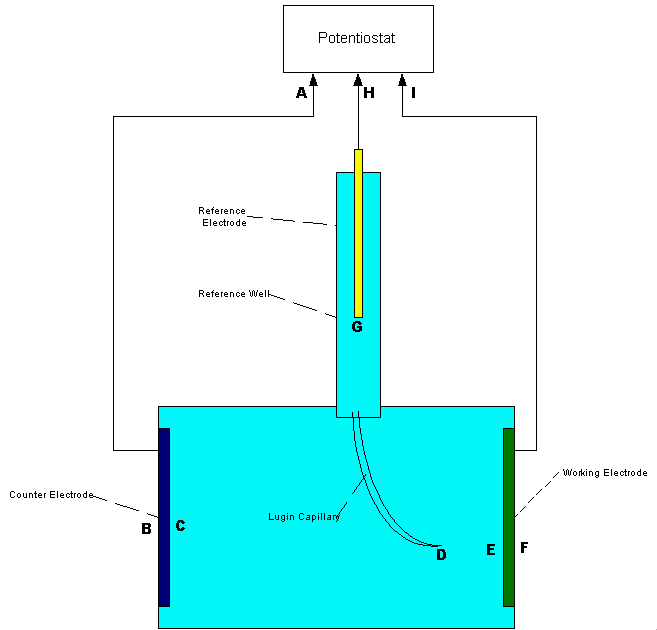
In plain words this drawing shows a piece of steel called 'the working electrode' corroding in an electrolyte. The corrosion current passes through the electrolyte to the other piece of steel called the 'counter electrode'.
The potential of each piece of steel is regulated by a device called a 'potentiostat'. The 'reference electrode' is shown to look like a half-cell with a copper rod 'reference electrode' in a saturated solution of copper sulphate 'reference well'.
The Lugin Capillary is a fine glass tube filled with conductive gell that provides a chemically inert conductive path to an exact position in the active region of the interface between the working electrode and the electrolyte.
Even this sophisticated arrangement leaves some sceptisism due to the fact that making the measurement disturbs the value of the data it yields.
There have been some attempts to apply the principles of this type of measurement in the field by Global Cathodic Protection in Libya for example. A plastic tube is provided to represent the lugin capillary but of course this tube finishes at the coating and not at the bare metal interface that would be present at a coating fault.
Such arrangements are of no technical or scientific value.
There are several arrangements of electrodes patented by scientists in an attempt to resolve this problem. CPN has one such device available known as the Isopotential Cell and will be described in detail elsewhere in this course.
If we cannot make the same measurements in our work then we cannot apply the equations. The Alexander Cell is the only available method to obtain the correct values in field work, to apply to the Pourbaix Diagrams.
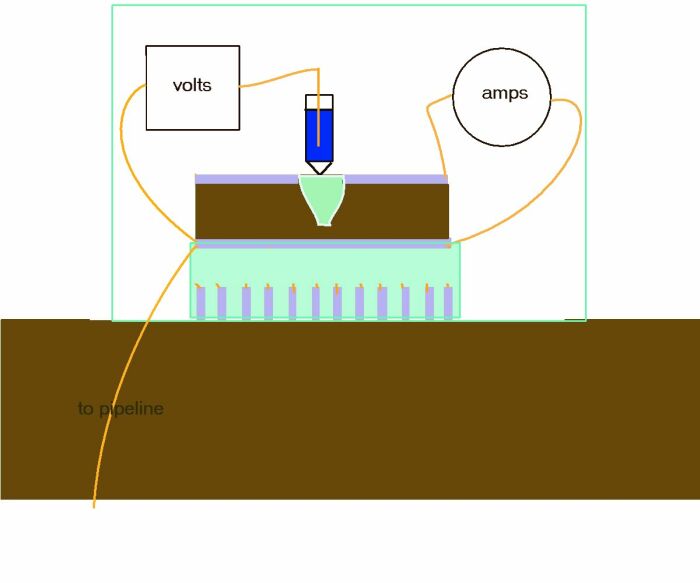

This will be made clear during this course.
