
Cathodic Protection Training Course
Module 3
Standard laboratory techniques

Standard Laboratory Techniques allow scientists all over the world to co-operate in the general advance of science and understanding.
Standard tests are carried out to ensure accurate product data can be published and that the quality of products conform to the specification required by the consumer.
However, we should not rely on the scientific establishment to provide absolute solutions to pratical problems
The following is my reason for doubt.
During a visit to UMIST I was shown a glass tank that was set up to conduct a 'cathodic disbondment test' intended to conform to the BSI code of practice.
Specified positioning of the test coupons in relation to the reference electrode had been ignored and readings were being adjusted by controlling the anode potential level.
The test was ineffective, but the reported results would contain enough of the right numbers to keep the client (a major paint manufacturer) happy and to convince the paint users that the BSI standard had been met.
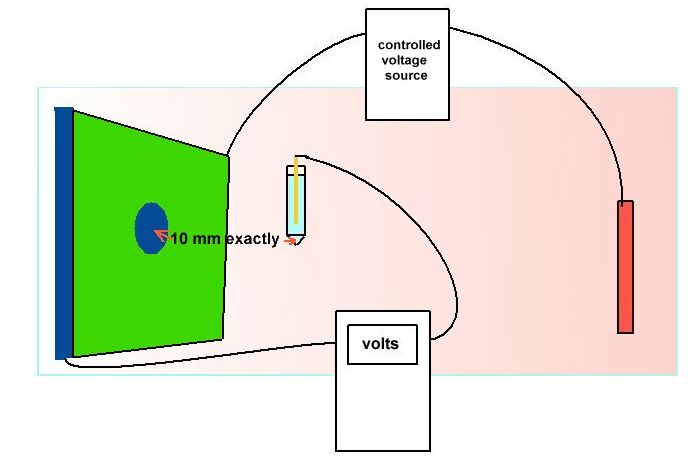
Prof. Les Wolff wrote this particular BSI code of practice and had supported me (in writing) relating to my publication about the problems that are inherent in cathodic protection field work. He was specific about the positioning of the reference electrode which should have been EXACTLY 10mm from the metal/electrolyte interface.
There is much more to this matter than is apparent from a purely scientific point of view.
UMIST was recognised as an accademic institution capable of carrying out scientific test for industry who had to pay them for results that would allow them to sell their products on the international markets.
The way these tests are conducted are up to individuals who had 'peer approval' and pieces of paper to say that they are competent. I recognise that it is necessary to have some discipline and structure in place but it is also recognised by science that hypotheses and theries must be supported by repeated observations.
The internet now makes it possible for serious specialists to offer their proposals and claims to the global audience for 'peer review' and this is resulting in cathodic protection becoming understandable to any student of any or no academic background.
The matter of cathodic disbondment can now be examined by experiment and observations in the field and in bench tests using science based reasoning. In this drawing we see that the electrical charges passing from the electrolyte in the test tank become directional as they approach the whole cut in the coating on the metal coupon.
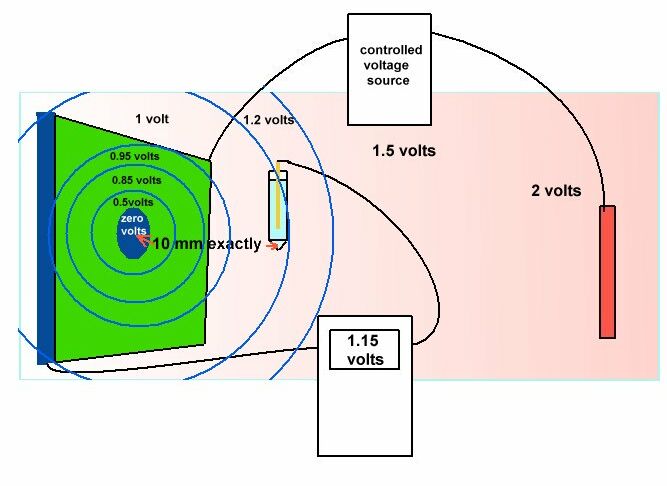
The metal of the coupon itself is at zero in this voltage measurement and the shells of resistance surrounding the whole in the coating form layers of potential that can be measured between two probes.
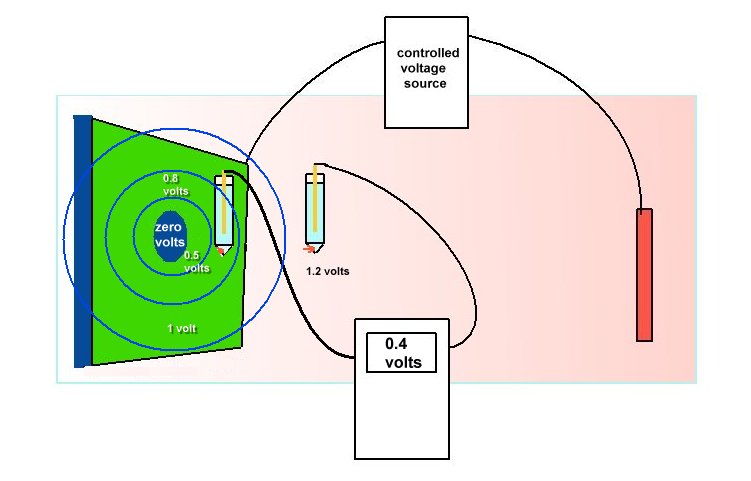
However, there are scientists working for coating manufacturers who really do understand and relate laboratory work to field experience. These scientists invariably question the concept of cathodic disbondment as the recommended tests reveal flawed logic.
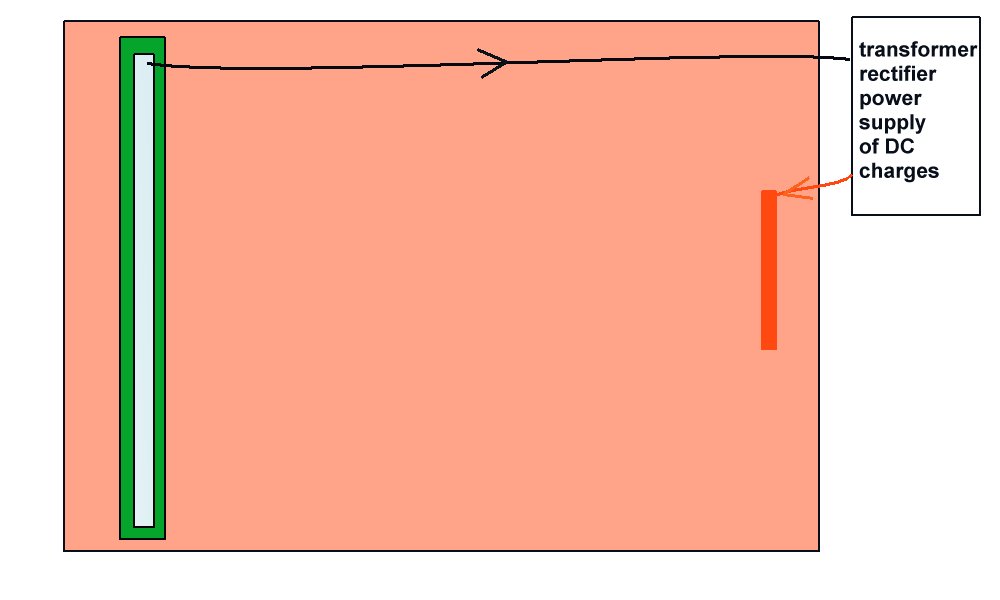
In this picture you can see that the metal coupon is completely cavered with very highly resistant coating. The charges flow into the electrolyte but not into the metal to complete the circuit. There can be no cathodic disbondment as there is no current flowing to release the hydrogen that is believed to blow the coating off.
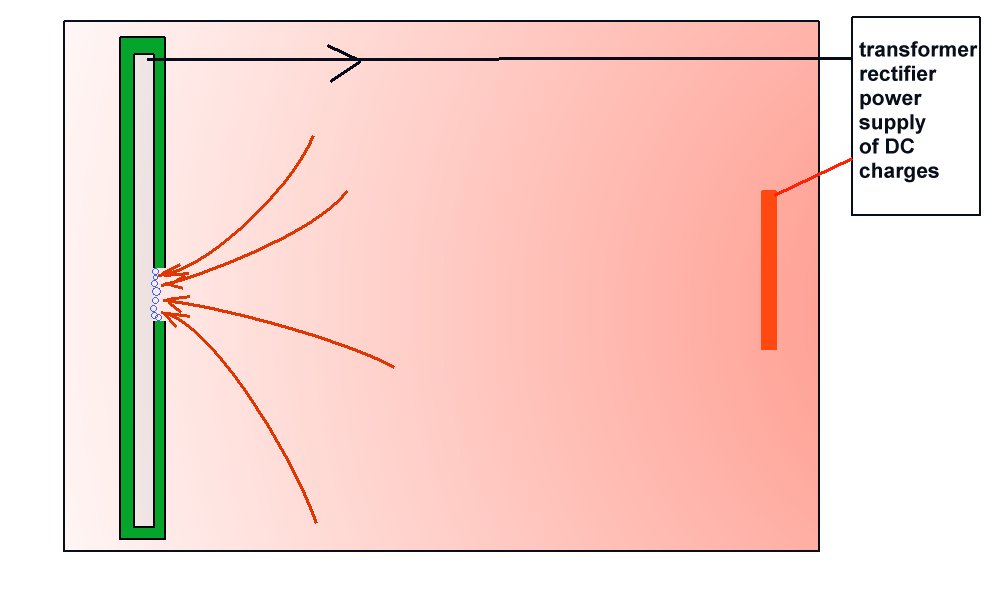
However, if there is a hole in the coating then current will flow and hydrogen will be formed. It will not blow the coating off as there is not coating!!
I have met many specialists in other countries who have excellent laboratories that they have developed themselves. Their tests match exactly the codes of practice and recommendations gleaned from international publications
At a laboratory in South Africa I saw a properly arranged cathodic disbondment test where the coating did not disbond but melted when 25 volts sent enough current through the electrolyte close to the hole to generate sufficient heat to melt the coating. Estimated temperature several hundred degrees.
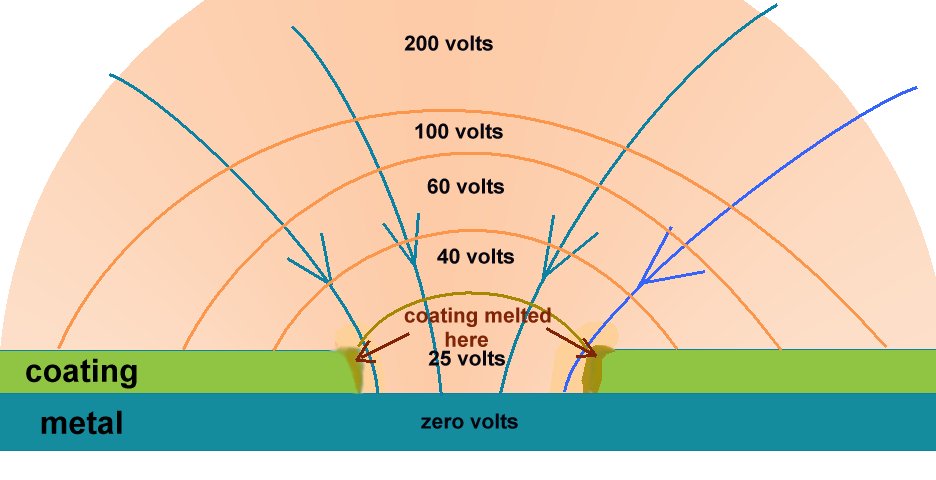
The total voltage in the system was several hundred volts and this must be compared with the total voltage in any field CP system. It there were such voltages in a pipelines situation it would certainly electrocute any passing animal or person. The whole notion is laughable.
It is vitally important for corrosion engineers to understand that field conditions are not readily mirrored in the laboratory
The purpose of CPN is to stop corrosion.
If our custommers want corrosion stopped they will have to come to us as most competition is only interested in is making money.
During my travels as a corrosion engineer all of my client have said that what they have learnt at university has not worked in the field.
back to Module03 index page