Cathodic Protection Network Two Day Seminar
This seminar is intended to draw the attention of the corrosion control community to the basis of all cathodic protection theory
and that this is NOT how it is put into practice.

Visit to Iran 18th - 27th November 2014
22nd November 2014 acquiring materials fo demonstrations..... only managed the three nails, batteries 3 half-cells, several mutlimeters in technical training centre.
Met the director of the training centre Sahm and 'clicked'. Set up most of the requirements for the seminar.
23rd November 2014 Planned start 0800actual start 0915.
Periods overan and overlapped but successful. 50% disturbed.
24th November 2014 Start 0830 each period dealt with and maybe 90% converted to following through.
Among those present were the following.
Omid Haghighi
Head of Quality Mangement System,
Esfahan Technical and Specialised Training Centre.
Javav Khosravi Shahmirzadi
Project Manager of Material and Corrosion Engineering
Sayed Abolfazi Hosseini Moghaddam PhD
Director
Esfahan Technical and Specialised Training Centre.
J.Khosravi Shahmirzadi(M.Sc)
ICA Vice President
Iranian Corrosion Association.
TWI Turkey
S.K.Alavi
NIOC Training Manager
32 students attended and probably over 20 asked for my email address.
25th November 2014
NIOC
Corrosion Control Conference
Esfahan,
Met many senior people who want me to come back and help them.
The paper was about the need for an international standard potential and they wanted to know how to go about it.
Also many reactions to the half-cell not being a reference electrode and interest in the Alexander Cell being a criterion.
This visit could be a turning point in the science and engineering of cathodic protection as the people attending the two day seminar seem now to be reconsidering the axioms that they have been taught.
The first thing that I tried to establish is that we are universally governed by the laws of nature and that these rules are being codified by science and are absolute.
This is the reason why it is essential to demonstrate the facts using the three nails experiment at the beginning of all basic discussions about corrosion control.
The main point of the seminar is that the present criteria of -0.850v, 300mv shift and 100mv polarisation, are not soundly based in science and NACE cannot be sure of it's publications because it attaches legal disclaimers to all it's advice.
There was considerable resistance to change during the introduction period and first periods of the seminar but this gradually converted to concern for an alternative criterion.
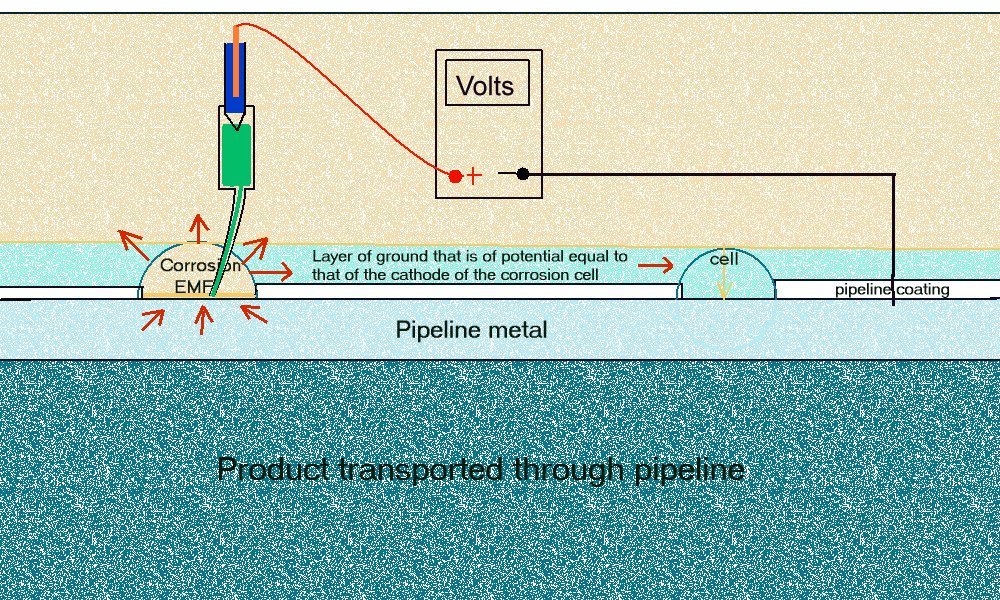
It was agreed at the seminar that the 'pipe-to-soil potential' measurement is a voltage between two variable potentials, and that this voltage did not have a common zero for the purposes of computation or electrical design.
This means that we cannot measure the electrical equilibrium when we have stopped corrosion using a copper/copper-sulphated electrode and that all the historical records must be re-assessed using a corrosion cell in which we can measure the corrosion current in closed circuit condition. This arrangement must allow the cathodic protection systems and other influences to affect the corrosion reaction as it does on corrosion cells on the actual pipeline.
The delegates at the seminar understood that the Alexander Cell is a corrosion cell that allows the measurement of corrosion current between the anode and the cathode. It also allows the influence of the cathodic protection system to be tested to see that corrosion has been controlled.
The seminar accepted that presently available software is simply graphic display of voltages that have to be interpreted using assumptions that are not soundly based.
There are a gathering number of software developers who are interested in the CPN Dynamic Software Project that will lead to the automatic control of corrosion on networks of pipelines. The seminar accepted that this is possible using the Alexander Cell as the trigger to adjust the settings of the sources of charges into the system. (TR's and controlled sacrificial anodes)
Remote monitoring is now commonplace but it has no way of telling if corrosion has been controlled. The term 'protected' is used in the sales of remote monitoring systems to mean that the present criteria are achieved but it was agreed by the seminar that this does not necessarily mean that corrosion has been controlled.
The delegates realise that cathodic protection systems can cause accelerated corrosion if wrongly applied and this is confirmed by the three nails demonstration.
It was accepted that the world is now one cathodic protection network of metalic paths and electrical influences that affect the corrosion to all pipelines.
It was also agreed that scientific knowledge is not successfully applied in the field because of poor exchange of knowledge and data.
It was also accepted that there is no such thing as zero potential and that all cathodic protection measurements are made referring to the zero that is set by the meter at the time of the measurement.
The result of this misunderstanding is that pipelines are leaking unnecessarily all over the world. It is therefore essential to be clear about the measurement we are making at present and the measurement that is required by all scientists. That is why this seminar is best attended by academics and field workers who can confirm all of the content. It is why the policy makers must be clear about the advice they are given and the effect of the decisions they make.
During Period 1 we examined the dry cell battery as a corrosion cell and compared it to a corrosion cell on a pipeline. We examined the measurement of the EMF of a dry cell battery and that of a corrosion cell on a pipeline using the 'pipe-to-soil potential' method using a copper/copper-sulphate electrode.
We observed that there are many corrosion cells on any span of pipeline and that we are measuring the combined effect of all these reactions on the potential of that complete metalic block. This is shown by the use of battery holders that configure many batteries in many ways.
We refer back to the experiment using three nails to make the following observation.
It is quickly apparent that the measuring circuit has more than two potentials in series because the readings change when the electrode is moved. The negative of the meter is permanently attached to the negative pole of the battery and the other is permanently attached to the electrode that we are told to regard as a reference potential.
The only change in the measuring circuit is the location of the electrode point of contact so it must be that each location itself has a different potential. We are measuring the potential of the ground itself, and this is an unknown variable. The voltage on the display is therefore between the pipeline metal/electrolyte potential and the copper/copper-sulphate reaction potential AND whatever other influences affect the potential of the ground on which the electrode is placed. This means that the data cannot be analysed mathematically or on a computer without further data.
The term ‘half-cell reaction’ was wrongly taken to infer that you could make a ‘half-cell’ and use it as a reference against which you can measure another potential. In fact this can only be done in controlled circumstances in a ‘closed circuit measurement’ condition but the demonstration (involving the three nails) proves conclusively that you cannot do this in field work.
The ‘criterion’ for achieving cathodic protection is widely accepted to be a voltage measurement that is supposed to indicate an equilibrium between the metal and the electrolyte at which no metal is going into solution. No corrosion is taking place because the electrochemical reaction has been halted.
This criterion has been under continuous review for at least 50 years, that I know of, and there is still no advice that is backed by demonstrable evidence, published by NACE, ISO or any of the corrosion control institutions of which I am aware.
Alexander Cell has now been extensively field trialled and in 2009 I made presentations at two international conferences in Brazil, announcing it as a definitive criterion for cathodic protection.
I am often asked why the Alexander Cell is not in general use so I devoted the second period to the history of the development of the Alexander Cell and my personal experiences in CP.
When CP was first introduced, it proved so cost effective that it became very widely used before its field application was fully understood. A short term measuring technique was required to monitor the performance, and the half-cell measurement was found to give a satisfactory indication. Thermodynamic theory seemed to support this notion as the ‘half-cell reaction’ was mentioned when describing reference electrodes in laboratories.
Field technicians and engineers must have known, from the early years, that there was something wrong with the readings produced by the traditional pipe-to-soil potential measuring technique.
Pipe-to-soil potentials are the foundation of all CP work. The measuring techniques are still obscure and imprecise.
The first text book that I read on the subject was published in 1953, and some of the information it contained, simply did not work when applied in the field.
Many other books and publications that contain advice and information that does not yield the predicted results.
This encouraged me to conduct my own field research and experiments which I have continued since 1972.
The traditional technique for making the standard CP 'pipe-to-soil' became open to question when pipelines began to fail in spite of having been theoretically 'protected’.
This seminar is not merely destructive criticism, as methods to overcome the problem, that has been identified, are included in the discourse.
We need to measure energy transfer in a corrosion cell at each location that we subject to cathodic protection. (Coating faults)
We can then use the electrical equilibrium in potentials and the cessation of corrosion current flow to indicate when we have stopped corrosion.
We can use this as a criterion for cathodic protection in design, installation, commissioning and maintenance of our integrated corrosion control systems on networks of pipelines and facilities.
The second period ended with the suggestion that we need to computerise cathodic protection.
The third period dealt with the field use of the copper/copper-sulphate electrode and the attempts to make sense of the measurements.
The changes in instrumentation show that the corrosion control community were continuously trying to overcome a recognised error in the measurements.
The attempt to remove the error by switching off the impressed current system was the start of the use of the words 'polarised potential' and I have explained that this cannot be measured in the field due to the fact that we have multiple corrosion cells on one side of the measuring circuit. The scientists who I met were only capable of measuring the polarised potential when the measurement was made in closed circuit condition with a single corrosion cell. All of the attempts to make meaningfull 'off potential' measurements are a misunderstanding of the science.
I have documentation in support of the material I presented during period 3 and explained that my suggested solution to the problem was suppressed by commercial interests/
In period 4 I explained the importance of understanding exactly how the measuring instruments work as this is relevant to the development of applied cathodic protection.
Period 5 discusses equivalent circuits that differ from those proposed in reference manuals and it is suggested that these are made in the laboratory for use by NIOC training and research.
Period 6 discussed a further physical model that should also be made for training purposes and experimentation. This period leads into the proposal that it is possible to create a control room where data gathering and analysis can be viewed dynamically.
Period 7 discussed the need for a training and research centre similar to that in Brazil.
The second day was about the way forward from the present status.
Each of the periods is self explanatory and the proposed actions need synchronising and co-ordinating with NIOC and the other pipeline owners in Iran.
Corrosion control is impossible when there are competing interests.
Proposal.
It is proposed that the seminar notes are edited and circulated to all interested parties as part of the proposed manual that was subject of the conference.
A one week course should be organised to be attended by maintenance and corrosion engineers to introduce the revised concept of applied cathodic protection in line with real science.
Laboratory tests should be carried out to scrutinise all codes of practice and advice (including my own advice published as CPN technology).
Historical data should be gathered for examination and be available for the software development team.
A software development team leader should be appointed to review and consider the CPN Dynamic Software Project.
The Alexander Cell with instructions and training should be put into use in the field as well as tested in the laboratory.
A university department should be established in the field of applied cathodic protection and electronic corrosion control. This university in Iran would co-operate with the South African University that is being established and those in Nigeria and Brazil that are being briefed at this time. CPN has followers in many countries at top university and government research establishment level that can be co-ordinated and synchronised.
We must develop the automatic computerised control of corrosion on networks of pipelines and facilities as the only real way of addressing the known corrosion problems in Iran and Nigeria. I believe that every country has similar problems, some of which are actually caused by the wrong application of impressed current cathodic protection.
Iran is well placed to lead the world in these matters as it is an advanced civilisation with a respected academic establishment.

























